Abstract
Despite the crucial role played by cholesterol and copper in nutrition and normal brain function, recent evidence indicates that they may both be important factors in the etiology of Alzheimer's disease (AD). Here we provide critical evidence for the role of cholesterol and copper in AD by showing that the addition of trace amounts of copper (0.12 ppm) to water given to cholesterol-fed rabbits can induce β-amyloid (Aβ) accumulation, including senile plaque-like structures in the hippocampus and temporal lobe, and can significantly retard the ability of rabbits to learn a difficult trace conditioning task. The Aβ deposits do not affect the ability of rabbits to detect or respond to the training stimuli nor to learn a simpler delay conditioning task. Trace amounts of copper in drinking water may influence clearance of Aβ from the brain at the level of the interface between the blood and cerebrovasculature and combined with high cholesterol may be a key component to the accumulation of Aβ in the brain, having a significant impact on learning and memory. Cholesterol-fed rabbits have at least 12 pathological markers seen in AD, suggesting that the cholesterolfed rabbit is a good animal model for studying AD.
Cholesterol is important for cell function, neurotransmission, and synaptic plasticity (1, 2), but it is also an important risk factor for atherosclerosis and Alzheimer's disease (AD) (3, 4). Recent evidence suggests that the cholesterol-lowering statins may affect AD by reducing the deposition of β-amyloid (Aβ) (5, 6). Copper is an essential nutrient (7) but has also been implicated as an important factor in AD (8–11). Indeed, Aβ has been hypothesized to act as a bioflocculant that binds trace metals such as copper and zinc (12), and up-regulation of the Aβ precursor protein (APP) has been shown to suppress elevated copper levels (11). Consequently, chelation of copper has been suggested as a potential therapy for AD (9, 13–16).
Substantial evidence has accrued that cholesterol-fed rabbits develop a considerable number of pathological indices of AD, including accumulation of Aβ in the hippocampus and temporal lobe (17–27). We have recently found that there are between-laboratory differences in the levels of Aβ that accumulate in cholesterol-fed rabbits and that these differences are traceable to the drinking water (28). Facilities that used distilled water had cholesterol-fed rabbits with lower levels of Aβ than those that used tap water. Analysis of the water from locales where tap water produced this differential effect indicated that, among likely trace metal candidates implicated in AD pathology (8, 29), copper levels ranging from 0.06 to 0.21 ppm were present in the drinking water. We initiated studies to assess the biological and behavioral effects of copper by supplementing distilled drinking water given to cholesterol-fed rabbits with copper sulfate at levels within this range (0.12 ppm copper) but at one-tenth the Environmental Protection Agency maximum allowable contaminant level for copper in municipal drinking water (1.3 ppm) (30).
Materials and Methods
Subjects. A total of 68 male New Zealand White rabbits (Oryctolagus cuniculus) 3–4 months of age and weighing ≈2.2 kg were housed individually, with free access to Purina rabbit chow and water, maintained on a 12-h light/12-h dark cycle and treated following National Institutes of Health guidelines. Each of these rabbits received a combination of food and water comprising normal chow or normal chow plus 2% cholesterol and tap water, distilled water, or distilled water plus copper. The number of subjects assigned to each condition is shown in Table 1. Briefly, 19 of the 68 rabbits were fed normal chow and 49 were fed the normal chow plus 2% cholesterol. Of the 19 rabbits fed normal chow, 9 received tap water (Tap) and 10 received distilled water (dH2O). Of the 49 rabbits fed cholesterol, 16 received tap water (Tap/Chol), 21 received distilled water (dH2O/Chol), and 12 received copper in their distilled water (dH2O/Chol/Cu). Cholesterol-fed rabbits given copper received copper sulfate in their distilled drinking water with a final copper concentration of 0.12 ppm (0.12 mg/liter). All rabbits were maintained on their respective food and water regimes for a total of 10 weeks before euthanasia and subsequent histological analysis.
Table 1. Number of Aβ immunoreactive neurons in differing regions of rabbit brain.
| Treatment | Hippocampus | Hilus (center) | Temporal cortex | Parietal cortex | Superior cortex |
|---|---|---|---|---|---|
| Tap | 43.9 ± 12.6 | 15 ± 4 | 42 ± 8 | 18 ± 5 | 26 ± 5 |
| Tap/Chol | 49.4 ± 8.6 | 19 ± 5 | 72 ± 9* | 28 ± 5 | 59 ± 5* |
| dH2O | 31.2 ± 5.8 | 10 ± 3 | 29 ± 4 | 11 ± 3 | 27 ± 4 |
| dH2O/Chol | 48.3 ± 7.8* | 12 ± 3 | 58 ± 6* | 28 ± 6* | 49 ± 4* |
| dH2O/Chol/Cu | 64.9 ± 16.3* | 21 ± 4** | 87 ± 11** | 27 ± 5 | 88 ± 14** |
Tap, tap water and normal chow (n = 9); Tap/Chol, tap water and 2% cholesterol diet (n = 16); dH2O, distilled water and normal chow (n = 10); dH2O/Chol, distilled water and 2% cholesterol diet (n = 21); dH2O/Chol/Cu, distilled water with 2% cholesterol diet and 0.12 ppm CU as CuSO4 (n = 12). *, P < 0.05; **, P < 0.01 (Tap/Chol vs. dH2O; dH2O/Chol vs. dH2O; dH2O/Chol/Cu vs. dH2O).
Behavioral Procedures. The behavioral procedures and equipment have been described (31). After 8 weeks on their respective diets, six of the cholesterol-fed rabbits given distilled water (dH2O/Chol) and six of the cholesterol-fed rabbits given distilled water supplemented with 0.12 ppm copper (dH2O/Chol/Cu) received 1 day of adaptation; one 60-trial session of air puff testing (pretest) to assess sensitivity to air puff; eight daily sessions of trace classical conditioning to assess their ability to learn a difficult conditioning task; a 60-trial session of air puff testing (posttest) to test for any changes in sensitivity to air puff; four daily sessions of delay classical conditioning to test for their ability to learn a simple conditioning task; and 2 days of tone intensity testing during trace conditioning to assess their hearing. Each air puff test trial pretest and posttest involved the presentation of 1 of 15 possible combinations of stimulus intensity (0.5, 1.0, 2.0, 4.0, or 8.0 psi) and stimulus duration (25, 50, or 100 ms). Each trace conditioning trial consisted of a 100-ms, 1-kHz, 82-dB tone that was followed by a 500-ms trace interval of no stimulation and then a 100-ms, 4-psi air puff. Each delay conditioning trial consisted of a 400-ms, 1-kHz, 82-dB tone that coterminated with a 100-ms, 4-psi air puff. Tone intensity testing trials consisted of one of seven, 100-ms tone intensities (60, 65, 70, 75, 80, 85, or 90 dB) or a zero intensity (0 dB) followed by a 200-ms trace interval and then a 100-ms, 4-psi air puff. The tone intensities were presented as a random sequence eight times. All trials were delivered, on average, every 60 s (50–70 s range). Repeated-measures ANOVA was used to compare conditioned responses (CR) and unconditioned responses between the two groups.
Histology. Rabbits were anesthetized deeply with a mixture of ketamine (35 mg/kg) and xylazine (5 mg/kg), blood was drawn from the heart, and the rabbits were perfused transcardially with 0.5% paraformaldehyde. Brains were extracted and postfixed for 14 days in 4% paraformaldehyde. Fifty-micrometer vibratome sections of hippocampus and hippocampal cortex of the brain were immunostained with an antibody to Aβ (10D5; provided by Dale Schenk of Elan Pharmaceuticals, San Diego) by using published peroxidase-antiperoxidase immunohistochemical methods (17).
Although we consider 10D5 immunohistochemistry to be the most consistent and reliable method of quantifying Aβ accumulation, a number of alternative labeling methods have confirmed that we are observing Aβ accumulation in the cholesterol-fed rabbits. In addition to 10D5, which recognizes the first 16 aa of the N-terminal sequence of Aβ, the 2H3 antibody (Athena Neurosciences, San Francisco) as well as appropriate negative controls have been used to study Aβ immunoreactivity in cholesterol-fed rabbits with copper in their drinking water (23). The antibody 2H3 recognizes the first 12 aa of the N-terminal sequence of Aβ as well as the last 12 aa of the C-terminal sequence of secreted APP. This antibody was compared with 8E5 (Athena Neurosciences), which is specific for amino acids 444–592 of the N-terminal sequence of secreted APP. The immunohistochemistry showed significant immunoreactivity to 2H3 in the hippocampus and temporal cortex of cholesterol-fed rabbits with copper in the drinking water, and no detectable levels of 8E5. Thus, the staining observed in the brain tissue was of Aβ and not secreted APP (23). The 2H3 staining of Aβ reported by D.L.S. (23) was indistinguishable from previous and present 10D5 staining of Aβ. D.L.S. (25) reported europium immunoassays for Aβ1–42 with polyclonal antibodies performed on fresh-frozen brain tissue from cholesterol-fed rabbits with copper in their drinking water. The immunoassays found elevated concentrations of total Aβ in the temporal cortex compared with normal-diet controls. Finally, both immunohistochemistry with the 6E10 monoclonal antibody for Aβ and quantification of Aβ with a europium immunoassay have appeared in an independent report (26), further corroborating the accumulation of Aβ in cholesterol-fed rabbit brain.
Immunoreactive neurons were counted as described (28). In brief, the number of Aβ immunoreactive cells within 10 randomly chosen 0.5 × 0.5-mm square fields were counted at ×20 magnification and averaged by an experimenter blind to the treatment of the rabbits. The average numbers of cells for all rabbits were subjected to ANOVA and then to follow-up pairwise comparisons, which were performed between the distilled water normal chow (dH2O) control condition and the separate treatment groups.
Blood samples were assayed for superoxide dismutase (SOD; a copper/zinc free radical scavenger enzyme) and glutathione peroxidase (GpX; a peroxide detoxifying enzyme) as described (24), and ceruloplasmin (CP; a copper chaperone) was measured according to published methods (32). The average levels of SOD, GpX, and CP for all rabbits were subjected to ANOVA and then to follow-up pairwise comparisons, which were performed between the distilled water normal chow (dH2O) control condition and the separate treatment groups.
SOD and glutathione levels have been reported for fresh-frozen brain tissue taken from cholesterol-fed rabbits with cooper in their drinking water (23). There was a significant increase in SOD in the hippocampus and a suggestion of an increase in the temporal cortex of cholesterol-fed rabbits with copper in their drinking water relative to controls. Although there was a suggestion of a reduction in the levels of glutathione in cholesterol-fed rabbits with copper in their drinking water, there was no significant difference in either area (23).
Results
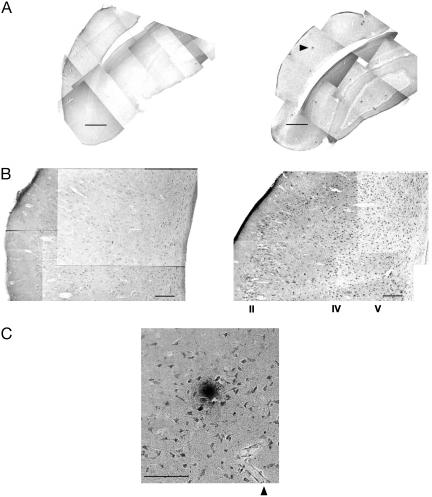
Copper Induces Aβ Accumulation and Senile Plaque (SP)-Like Structures. The brains of rabbits were analyzed by using the 10D5 antibody, which shows Aβ immunoreactivity (33, 34). Cholesterol-fed rabbits with copper (0.12 ppm) added to their distilled drinking water (dH2O/Chol/Cu) revealed a nearly 50% increase in the number of Aβ immunoreactive neurons compared with animals on a cholesterol diet and unaltered distilled water (dH2O/Chol) (Table 1). The hippocampus, temporal cortex, and superior parietal cortex were preferentially affected compared with lateral parietal cortex, an observation that is similar to the regional distribution of SPs in AD. The superior temporal gyrus, also severely affected in AD, demonstrated a laminated distribution of Aβ immunoreactive neurons (Fig. 1B). This propensity for Aβ immunoreactive neurons to occur in layers II and IV–V is reminiscent of the laminar distribution of SPs in layers II and IV–V of the AD temporal cortex (24, 35). We also noted an increased Aβ immunoreactive staining on the meningeal surface of the brain in rabbits supplemented with copper (Fig. 1 A Right and B Right), consistent with observations in the AD brain (36). Perhaps most significantly, cholesterol-fed rabbits also showed extracellular SP-like deposits of Aβ immunoreactive material (Fig. 1 A and C). These SP-like deposits were not found among animals given distilled water (dH2O/Chol), and rarely occur among animals on tap water (Tap/Chol, <10%; one or two per section in 1 of 16 animals). Strikingly, 75% of the cholesterol-fed animals on distilled water supplemented with 0.12 ppm copper (9 of 12) displayed SP-like deposits, and three of these rabbits had >10 SP-like deposits per histological section (Fig. 1 A).
Fig. 1.
Trace amounts of copper induce Aβ immunoreactive neurons and SP-like structures in the brain of the cholesterol-fed rabbit. (A) Photo montage of temporal lobe and hippocampus for a cholesterol-fed rabbit given distilled water (Left) and distilled water supplemented with 0.12 ppm copper (Right) (scale bar, 1 mm). Note the numerous dark spots in the copper-supplemented rabbit; these are SP-like structures. An example is indicated by the arrowhead and is magnified in C. (B) Photo montage of superior temporal gyrus for distilled water (Left) and copper-supplemented rabbit (Right) with banding of Aβ immunoreactive neurons in layers II and IV–V (scale bar, 100 μm). (C) SP-like structure surrounded by Aβ immunoreactive neurons. Note that the plaque-like structure is somewhat removed from a blood vessel indicated by the arrowhead (scale bar, 100 μm).
Copper and Cholesterol Increase SOD and Decrease GpX. We sought to find a bloodborne marker related to elevated circulating cholesterol or altered copper levels that might co-vary with Aβ immunoreactive neurons and/or SP-like deposits in the cholesterol-fed rabbit. Free radicals and associated changes of SOD and GpX activities are known to accompany increased circulating cholesterol (37), and CP can vary with copper levels in the blood (38). Using rabbits on distilled water as the reference control (dH2O), the activity of SOD in rabbits fed normal chow and given tap water (Tap) was marginally increased in RBCs and unchanged in the plasma (Table 2). Addition of cholesterol to the diet of animals on distilled water (dH2O/Chol) produced a 3-fold increase in SOD activity in the plasma, and the addition of 0.12 ppm copper (dH2O/Chol/Cu) produced an almost 4-fold increase in SOD activity (Table 2). Among the cholesterol-fed rabbits given copper sulfate in their distilled water, the activity of GpX decreased significantly compared with cholesterol-fed animals given distilled water. Although there was a suggested increase in CP, there was no significant effect of circulating cholesterol levels, concentration of copper in the drinking water, and levels of a CP in rabbit blood.
Table 2. Rabbit blood levels of SOD, GpX, and CP.
| Treatment | RBC SOD | Plasma SOD | RBC GpX | Plasma GpX | Plasma CP |
|---|---|---|---|---|---|
| Tap | 58 ± 4.6 | 0.57 ± 0.03 | 1.73 ± 0.17 | 7.4 ± 0.95 | 21 ± 1 |
| Tap/Chol | 52 ± 3.7 | 1.87 ± 0.23 | 1.73 ± 0.19 | 9.3 ± 0.71 | 82 ± 42 |
| dH2O | 49 ± 3.5 | 0.55 ± 0.05 | 1.97 ± 0.17 | 8.3 ± 0.87 | 30 ± 8 |
| dH2O/Chol | 48 ± 4.5 | 1.69 ± 0.26 | 1.64 ± 0.25 | 8.8 ± 0.77 | 47 ± 17 |
| dH2O/Chol/Cu | 40 ± 5.9 | 2.05 ± 0.49* | 0.88 ± 0.06* | 4.9 ± 1.2* | 78 ± 34 |
P < 0.05 (dH2O/Chol/Cu vs. dH2O).
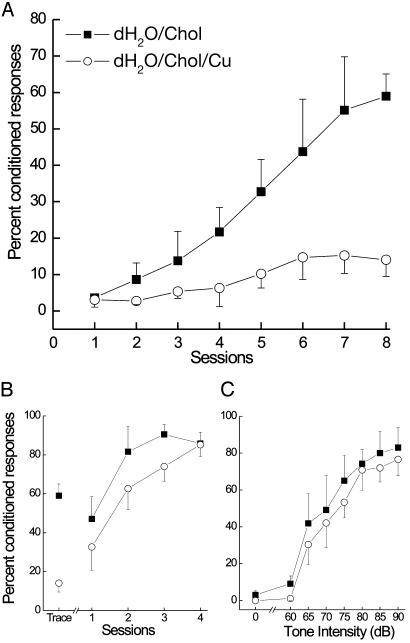
Copper-Induced Aβ Retards Trace Conditioning but Not Delay Conditioning. To assess the effects of copper-induced Aβ accumulation on learning, memory, and sensory processing, we devised and administered a battery of behavioral tests to a subset of the cholesterol-fed rabbits. The tests included a measure of sensitivity and responsiveness to air puff, a difficult learning task, an easy learning task, and a task designed to measure their hearing. We chose trace classical conditioning of the rabbit nictitating membrane response as the difficult learning task because it engages the hippocampus and temporal lobe (39, 40), areas in which we noted the highest levels of Aβ accumulation. Measures of the average percent CR to the tone across 8 days of hippocampally dependent tone–air puff trace conditioning showed that 0.12 ppm copper had a profound effect on the ability of cholesterol-fed rabbits to acquire CR (Fig. 2A). In fact, rabbits with copper in their drinking water (dH2O/Chol/Cu) did not exceed an average level of 15% CR, whereas rabbits on distilled water (dH2O/Chol) acquired CR approaching average levels of 60%. In strong contrast, when rabbits given copper were shifted to a simpler, hippocampally independent, short-delay conditioning paradigm, they were able to acquire CR to levels approaching 80% (Fig. 2B). Equally important, all rabbits showed comparable levels of sensitivity and responsiveness to the air puff and to the tone. Specifically, there were no significant differences between the copper-supplemented and distilled water groups in any of the dependent variables used to assess sensitivity and responsiveness to air puff, including response frequency, amplitude, latency, or area (P values >0.07). An analysis of hearing measured as percent CR to seven different tone intensities (60–90 dB) indicated that there were no between-group differences in the levels of responding to the tone intensities (P values >0.49) (Fig. 2C).
Fig. 2.
Effects of trace amounts of copper (0.12 ppm) induce deficits in trace but do not delay classical conditioning of the rabbit nictitating membrane response. (A) Trace conditioning. Mean (± SEM) percent CR for cholesterol-fed rabbits with distilled water (dH2O/Chol) and with copper sulfate (dH2O/Chol/Cu) added to their distilled water revealed a significant overall copper-induced learning deficit as a main effect of groups [F (1,9) = 7.37, P < 0.05] and a highly significant groups–sessions interaction [F (7,63) = 4.81, P < 0.001]. (B) Delay conditioning of the rabbit nictitating membrane response showed that there was no difference in rate or level of learning of a simpler task between rabbits given copper in their distilled water or distilled water alone. (C) Tone intensity testing after delay conditioning showed no differences in the ability of rabbits given copper in their distilled water or distilled water alone to hear the tone used during trace conditioning.
Discussion
Copper and AD. The present data provide strong support for the suggestion that copper is implicated in the accumulation of Aβ (8, 10). A recent report described APP as an exporting system for copper and suggested that elevated levels of APP in the brain may remove increased copper (11). Indeed, as we have already noted, chelation of copper that would prevent Aβ accumulation has been suggested as a potential therapy for AD (9, 13–15). Clioquinol, a copper–zinc chelator, is in phase I (13) and phase II clinical trials (16). In contrast to Aβ's potential neuronal role in clearing copper, copper may influence clearance of Aβ from the brain at the level of the interface between the blood and cerebrovasculature.
The Environmental Protections Agency maximum contaminant level goal (MCLG) for copper in drinking water is set at 1.3 ppm (1.3 mg/liter) copper or corrosives liberating 1.3 ppm copper, and treatment plants that exceed this MCLG are required to monitor the water and implement techniques that will reduce its corrosiveness (30). The MCLG is based on the lowest observed adverse health effect level (LOAEL) of 5.3 mg/day, a level which may induce gastrointestinal distress (30). Interestingly, although the dietary reference intake [DRI, formerly called recommended daily allowance (RDA)] for copper in adults is only 0.9 mg/day, the normal tolerable upper limit is considered to be 10 mg/day (7). Cholesterol-fed rabbits in the present experiment were allowed 0.12 ppm or 0.12 mg/liter, and given that rabbits drink ≈300–600 ml of water per day, they consumed between 0.04 and 0.08 mg of copper per day. The present data show that this level of copper consumption was sufficient to induce significant Aβ accumulation and the formation of significant SP-like structures in the cholesterol-fed rabbit. Although we can only speculate about how the effects of copper consumption in cholesterol-fed rabbits relate to those in humans, it is of note that the levels of copper in the cholesterol-fed rabbit drinking water that induced Aβ and SP-like structures are well below those considered safe for humans.
A Cholesterol-Fed Rabbit Model of AD. Unlike transgenic mouse models, which have failed to produce more than one or two signs of AD pathology (41), the cholesterol-fed rabbit shows pathologies consistent with at least 12 different features of the AD brain. These include the currently described neuronal accumulation of Aβ immunoreactivity (17, 19), extracellular Aβ plaques (23), and meningeal Aβ immunoreactivity (36), as well as previously noted apolipoprotein E immunoreactivity (18, 22), cathepsin D immunoreactivity (20, 23), SOD immunoreactivity (23), microgliosis (21, 23), apoptosis (21), vascular activation of SOD (24), mouse endothelial cell antigen (MECA-32) immunoreactivity (25), breaches of the blood brain barrier (25), elevated brain cholesterol (23), and elevated Aβ concentration (24). To these indices of pathology we can now add a deficit in the ability to learn a difficult task that depends on the temporal cortex and hippocampus.
Conclusions
The addition of trace amounts of copper to the drinking water of cholesterol-fed rabbits induces accumulation of Aβ, formation of SP-like structures, reduction of GpX activity, increases in SOD activity, and retardation of the rabbit's ability to learn a difficult task. Thus, there may be a relationship between the diminished ability to inactivate peroxide due to reduced GpX activity in the blood and increased neuronal accumulation of Aβ immunoreactivity, formation of SP-like structures in the neuropil, and observed deficits in complex memory acquisition. Overall, we would suggest that cholesterol entering the brain from the circulation of cholesterol-fed rabbits induces the neuronal accumulation of Aβ, and that copper influences clearance of Aβ from the brain at the level of the interface between the blood and cerebrovasculature.
Acknowledgments
We thank C. Smith-Bell and J. Lochhead for assistance with histology, data collection, and analysis. This research was supported by funds from the Blanchette Rockefeller Neurosciences Institute and the Arizona Disease Control Research Commission.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Aβ, β-amyloid; APP, Aβ precursor protein; CP, ceruloplasmin; CR, conditioned responses; GpX, glutathione peroxidase; SOD, superoxide dismutase; SP, senile plaque.
References
- 1.Goritz, C., Mauch, D. H., Nagler, K. & Pfrieger, F. W. (2002) J. Physiol. (Paris) 96, 257–263. [DOI] [PubMed] [Google Scholar]
- 2.Koudinov, A. R. & Koudinova, N. V. (2001) FASEB J. 15, 1858–1860. [DOI] [PubMed] [Google Scholar]
- 3.Sparks, D. L., Martin, T. A., Gross, D. R. & Hunsaker, J. C., III (2000) Microsc. Res. Tech. 50, 287–290. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann, T. (2001) Trends Neurosci. 11, S45–S48. [DOI] [PubMed] [Google Scholar]
- 5.Buxbaum, J. D., Cullen, E. I. & Friedhoff, L. T. (2002) Front. Biosci. 7, a50–a59. [DOI] [PubMed] [Google Scholar]
- 6.Fassbender, K., Stroick, M., Bertsch, T., Ragoschke, A., Kuehl, S., Walter, S., Walter, J., Brechtel, K., Muehlhauser, F., von Bergmann, K., et al. (2002) Neurology 59, 1257–1258. [DOI] [PubMed] [Google Scholar]
- 7.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (2001) Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc: A Report of the Panel of Micronutrients, Subcommittees on Upper Reference Levels of Nutrients (Natl. Acad. Press, Washington, DC).
- 8.Atwood, C. S., Moir, R. D., Huang, X., Scarpa, R. C., Bacarra, N. E., Romano, D. M., Hartshorn, M. A., Tanzi, R. E. & Bush, A. I. (1998) J. Biol. Chem. 21, 12817–12826. [DOI] [PubMed] [Google Scholar]
- 9.Bush, A. I. & Tanzi, R. E. (2002) Proc. Natl. Acad. Sci. USA 99, 7317–7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White, A. R., Multhaup, G., Galatis, D., McKinstry, W. J., Parker, M. W., Pipkorn, R., Beyreuther, K., Masters, C. L. & Caputo, C. (2002) J. Neurosci. 22, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maynard, C. L., Cappia, R., Volitakis, I., Cherny, R. A., White, A. R., Beyreuther, K., Masters, C. L., Bush, A. I. & Li, Q.-X. (2002) J. Biol. Chem. 277, 44670–44676. [DOI] [PubMed] [Google Scholar]
- 12.Robinson, S. R. & Bishop, G. M. (2002) Neurobiol. Aging 23, 1051–1072. [DOI] [PubMed] [Google Scholar]
- 13.Regland, B., Lehmann, W., Abedini, I., Blennow, K., Jonsson, M., Karlsson, I., Sjogren, M., Wallin, A., Xilinas, M. & Gottfries, C. G. (2001) Dement. Geriatr. Cognit. Disord. 12, 408–414. [DOI] [PubMed] [Google Scholar]
- 14.Cherny, R. A., Atwood, C. S., Xilinas, M. E., Gray, D. N., Jones, W. D., McLean, C. A., Barnham, K. J., Volitakis, I., Fraser, F. W., Kim, Y.-S., et al. (2001) Neuron 30, 665–676. [DOI] [PubMed] [Google Scholar]
- 15.Opazo, C., Barria, M. I., Ruiz, F. H. & Inestrosa, N. C. (2003) Biometals 16, 91–98. [DOI] [PubMed] [Google Scholar]
- 16.Bush, A. I. (2003) Trends Neurosci. 26, 207–214. [DOI] [PubMed] [Google Scholar]
- 17.Sparks, D. L., Scheff, S. W., Hunsaker, J. C., III, Liu, H., Landers, T. & Gross, D. R. (1994) Exp. Neurol. 126, 88–94. [DOI] [PubMed] [Google Scholar]
- 18.Sparks, D. L., Liu, H., Gross, D. R. & Scheff, S. (1995) Neurosci. Lett. 187, 142–144. [DOI] [PubMed] [Google Scholar]
- 19.Sparks, D. L. (1996) Neurobiol. Aging 17, 291–299. [DOI] [PubMed] [Google Scholar]
- 20.Haas, U. & Sparks, D. L. (1996) Mol. Chem. Neuropathol. 29, 1–14. [DOI] [PubMed] [Google Scholar]
- 21.Streit, W. J. & Sparks, D. L. (1997) J. Mol. Med. 72, 130–138. [DOI] [PubMed] [Google Scholar]
- 22.Sparks, D. L. (1997) Ann. N.Y. Acad. Sci. 826, 128–146. [DOI] [PubMed] [Google Scholar]
- 23.Sparks, D. L. (1997) Nutr. Metab. Cardiovasc. Dis. 7, 255–266. [Google Scholar]
- 24.Sparks, D. L. (1999) in Cerebral Cortex, eds. Peters, A. & Morrison, J. H. (Kluwer Academic, New York), pp. 733–772.10554996
- 25.Sparks, D. L., Kuo, Y.-M., Roher, A. E. & Martin, T. A. (2000) Ann. N.Y. Acad. Sci. 903, 335–344. [DOI] [PubMed] [Google Scholar]
- 26.Wu, C.-W., Liao, P.-C., Lin, C., Kuo, C.-J., Chen, S.-T., Cehn, H.-I. & Kuo, Y.-M. (2003) J. Neural Transm. 110, 641–649. [DOI] [PubMed] [Google Scholar]
- 27.Zatta, P., Zambenedetti, P., Stella, M. P. & Licastro, F. (2002) J. Alzheimer's Dis. 4, 1–9. [DOI] [PubMed] [Google Scholar]
- 28.Sparks, D. L., Lochhead, J., Horstman, D., Wagoner, T. & Martin, T. (2002) J. Alzheimer's Dis. 4, 519–525. [DOI] [PubMed] [Google Scholar]
- 29.Lovell, M. A., Robertson, J. D., Teesdale, W. J., Campbell, J. J. & Markesbery, W. R. (1998) J. Neurol. Sci. 158, 47–52. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Environmental Protection Agency (1994) Federal Register 59, 33860–33864. [PubMed] [Google Scholar]
- 31.Buck, D. L., Seager, M. A. & Schreurs, B. G. (2001) Behav. Neurosci. 115, 1039–1047. [PubMed] [Google Scholar]
- 32.Schosinsky, K. H., Lehmann, H. P. & Beeler, M. F. (1974) Clin. Chem. 20, 1556–1563. [PubMed] [Google Scholar]
- 33.Parvizi, J., Van Hoesen, G. W. & Damasio, A. R. (2001) Ann. Neurol. 49, 53–66. [DOI] [PubMed] [Google Scholar]
- 34.Bacskai, B. J., Kajdasz, S. T., McLellan, M. E., Games, D., Seubert, P. & Schenk, D. (2002) J. Neurosci. 22, 7873–7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beach, T. G. & McGreer, E. G. (1992) Acta Neuropathol. 83, 292–299. [DOI] [PubMed] [Google Scholar]
- 36.Shinkai, Y., Yoshimura, M., Ito, Y., Odaka, A., Suzuki, N., Yanagisawa, K. & Ihara, Y. (1995) Ann. Neurol. 38, 421–428. [DOI] [PubMed] [Google Scholar]
- 37.Erdincler, D. S., Seven, A., Inci, F., Beger, T. & Candan, G. (1997) Clin. Chim. Acta 265, 77–84. [DOI] [PubMed] [Google Scholar]
- 38.Araya, M., Olivares, M., Pizarro, F., Gonzalez, M., Speisky, H. & Uauy, R. (2003) Biometals 16, 199–204. [DOI] [PubMed] [Google Scholar]
- 39.Moyer, J. R., Jr., Deyo, R. A. & Disterhoft, J. F. (1990) Behav. Neurosci. 104, 243–252. [DOI] [PubMed] [Google Scholar]
- 40.Manns, J. R., Clark, R. E. & Squire, L. R. (2000) Learn. Mem. 7, 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bishop, G. A. & Robinson, S. R. (2002) Neurobiol. Aging 23, 1101–1105. [DOI] [PubMed] [Google Scholar]