Abstract
Adult owl monkeys were trained to detect an increase in the envelope frequency of a sinusoidally modulated 1-kHz tone. Detection was positively correlated with the magnitude of the change in the envelope frequency. Surprisingly, neuronal responses recorded in the primary auditory cortex of trained monkeys were globally suppressed by the modulated tone. However, the contrast in neuronal responsiveness to small increases versus large increases in envelope frequencies was actually enhanced in the trained animals. The results suggest behaviorally contingent inhibitory and excitatory processes that are modulated by the probability that a particular signal predicts a reward.
The primary auditory cortex (AI) is an important processing station for complex, biologically significant sounds. Primate vocalizations are represented and analyzed in the AI (1–3), and lesion studies indicate that the AI is essential for the recognition of communication sounds (4, 5). The temporal envelopes of mammalian vocalizations are typically amplitude-modulated at frequencies of 2–64 Hz (3, 6–8), a range of frequencies represented in AI neurons by their temporally synchronized discharge patterns (9–15). Temporal envelope cues are necessary and often sufficient for speech recognition in humans (16, 17) and are also required for normal AI neuronal responses to spectrally degraded vocalizations in monkeys (13).
This cortical field also expresses spectral and temporal response plasticity that includes experience-induced enhancement and suppression of AI neuronal responses (for reviews, see refs. 18–23). For example, during the learning phase of a frequency discrimination experiment, the response of AI neurons to a standard tone decreased and responses to target tones increased in awake owl monkeys (24). Spectral receptive fields in the AI also vary dynamically, increasing or decreasing their profiles to tones on a time scale of tens to hundreds of milliseconds in alert animals during differential behavioral conditioning (20, 21). These findings are consistent with theories of animal conditioning and learning that emphasize the importance of excitatory and inhibitory processes in behavior (for reviews, see refs. 25–32).
In the present study, owl monkeys were trained to discriminate a change in the envelope frequency of a sinusoidally amplitude modulated (SAM) tone. The behavioral task required an animal to execute a motor response to produce a reward (instrumental learning). After extensive training, AI neuronal responses to SAM signals were recorded in trained and naïve monkeys. The neuronal representations of signals that were infrequently or never paired with rewards during training were consistently suppressed. However, SAM signals that were frequently paired with rewards had relatively robust representations in AI. An important principle in contemporary animal learning theory is that classical (Pavlovian) inhibitory and excitatory conditioning processes modulate instrumental learning and performance by formation of associations between the central representations of conditioned and unconditioned stimuli (25, 29, 30, 32). Our results are consistent with the hypothesis that Pavlovian conditioning processes in animals trained instrumentally on a temporal discrimination task modified the neuronal representations of SAM signals in the AI.
Methods
This article is based on data obtained during the second of a two-stage experimental protocol. The first stage included discrimination training using SAM tones, followed by recording of AI neuronal responses in two trained monkeys. Temporal discrimination training with SAM tones continued in the second stage and ended with a redundant schedule of sessions using a SAM 1-kHz tone. Responses of AI neurons were then recorded in the two trained monkeys and in two untrained (naïve) monkeys. All procedures described followed National Institutes of Health and University of California, San Francisco guidelines for the care and use of laboratory animals.
Auditory Stimuli. Behavioral training and electrophysiological recording were conducted in sound-attenuated chambers lined with 7.6-cm Sonex foam (Illbruck, Minneapolis). SAM tones were produced by computer-controlled hardware (H-P 3312A function generator, Agilent, Palo Alto, CA; TMS 32010 microprocessor, Texas Instruments, Dallas), and the stimulus generation systems were calibrated with a sound level meter (B&K 2209, Brüell & Kjaer, Naerum, Denmark), a spectrum analyzer (NCS UA-500A, Nicolet Scientific, Northvale, NJ), and a microphone (B&K 4134, Brüell & Kjaer). The microphone was located 24 cm from a speaker (Realistic 40–1996A, Radio Shack, Fort Worth, TX) at the position occupied by a monkey's head during the psychophysical and physiological experiments.
In the physiological experiments, the stimuli were 1-s SAM tones. The modulated stimuli had two components: a standard envelope frequency (SAMs: 4 or 10 Hz, 500-ms duration) followed by a comparison envelope frequency (SAMc: 4–32 Hz or 10–40 Hz, equal logarithmic intervals, 500-ms duration). The free-field stimuli were delivered at a rate of 0.5 Hz (10 repetitions per modulation frequency). The stimulus level (65 dB-A sound pressure level) and the modulation depth (80%) were identical to those used in psychophysical training.
Psychophysical Procedures. On each trial, a trained monkey positioned its face against an acoustically transparent wire ring located 24 cm from the speaker to initiate the onset of a 1-kHz modulated tone. After a variable delay (1–3 s), the modulation changed randomly from a SAMs to one of six higher SAMc (psychophysical method of constant stimuli). If the monkey broke contact with the ring within1safterSAMc onset, a correct response (hit) and the reaction time (RT) were recorded, and a liquid reward was delivered at a spout adjacent to the wire ring. If a monkey responded during SAMs (false alarm, FA) or failed to respond during SAMc (miss), a 1- to 4-s timeout (TO) occurred (chamber light off, no reward). In one monkey (OM2178), SAMs was set at 4 Hz (30 sessions), and SAMc ranged from 5 to 18 Hz; in the second monkey (OM2184), SAMs was set at 10 Hz (26 sessions), and SAMc ranged from 11 to 24 Hz. Stimulus timing and recording of behavioral responses were controlled by a computer-based virtual instrument environment (Labview, National Instuments, Austin, TX).
The proportions of correct responses, p(HIT), at each comparison frequency were pooled from the final 15 sessions in which performance was clearly under stimulus control. Plots of p(HIT) and mean median RT versus SAMc yield psychometric functions (Fig. 1). The 50% detection thresholds in Hz were estimated by linear interpolation from the p(HIT) functions, and difference limens (threshold minus SAMs in H3) were calculated.
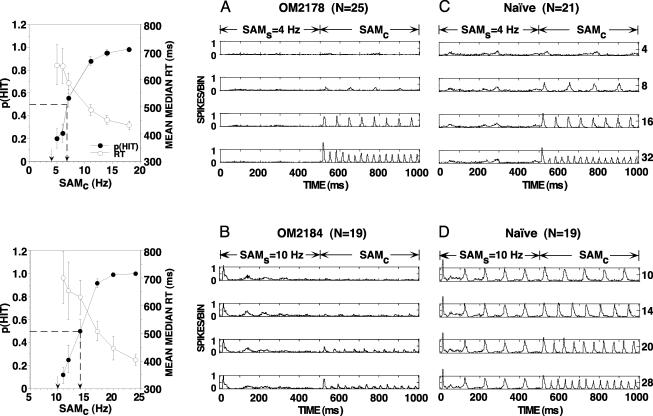
Fig. 1.
(Left) Mean p(HIT) and average RT versus SAMc for two trained monkeys. Error bars: SD (sessions). Arrows indicate SAMs (4 and 10 Hz) and 50% detection thresholds. (A–D) Compound PSTHs (spikes per bin) normalized for number of recording sites (N). Bin width: 2 ms. Neuronal CFs are within an octave band centered at 1 kHz. Carrier frequency: 1 kHz. PSTHs represent the population responses evoked by standard (SAMs;0–500 ms) and comparison (SAMc; 500–1,000 ms) modulation frequencies. Numbers to the right of PSTHs indicate SAMc frequencies (Hz). (A and B) Data for trained monkeys OM2178 (SAMs = 4 Hz) and OM2184 (SAMs = 10 Hz). (C and D) Data for naïve monkeys.
Physiological Procedures. Preparation. At the conclusion of behavioral training, a monkey was sedated (ketamine, 22 mg/kg), and anesthesia was induced by i.v. infusion of pentobarbital sodium (7–10 mg/kg). The animal's head was placed in a holder that permitted unobstructed sound delivery. The left AI was exposed, and the brain was protected with silicone oil. After recording at 40–70 densely sampled locations, the right AI was exposed for recordings at 25–60 additional locations. Anesthesia was maintained throughout the experiment by supplemental doses of pentobarbital sodium. Lactated Ringer's solution (4 ml/hr) was administered i.v., and rectal temperature, heart rate, and oxygen saturation level were monitored continuously and maintained at normal physiological levels.
Recording. Electrode penetrations were confined to the lateral surface region of AI (33, 34), with an emphasis on a four-octave band representational zone (0.35–5.6 kHz). Responses of single neurons and multineuronal clusters to SAM tones were recorded with single or double tungsten microelectrodes (1.3–1.5 MΩ at 1 kHz) at depths of 700–1,000 μm (layers 3–4) relative to the surface of the cortex. Responses were amplified (×10,000–20,000) and band-pass-filtered (0.3–10 kHz), and neuronal spikes were isolated with a window discriminator (DIS-1, BAK Electronics, Mount Airy, MD). The time of occurrence of each discriminated spike was stored in a computer.
Data analysis. The present article is based on neuronal responses to the SAM tone frequency (1 kHz) used at the end of behavioral training and includes data from all recording sites that met two criteria. First, the characteristic frequency (CF: the frequency that evoked a response at the lowest stimulus amplitude) at each site was within a 1-octave band centered at 1 kHz (0.707–1.414 kHz). CF and minimum threshold at CF were determined by using 50-ms duration pure tones and conventional recording procedures (34). Mean CFs and mean thresholds were not significantly different in trained versus naïve monkeys (P > 0.05). Second, each site was tested for responses to SAM tone frequencies of 0.5, 1, and 2 kHz. Recording sites that responded to the 1-kHz SAM tone are included in Results (OM2178, n = 25; OM2184, n = 19; naïve, n = 19 and 21).
Using customized software, neuronal responses at each recording site were analyzed quantitatively as functions of the SAM signals to produce two kinds of spike rate modulation transfer functions (MTFs): the phase-locked response to each modulation envelope frequency (total spikes × vector strength; pMTF) and the entrained response to each stimulus cycle (driven spikes per cycle; eMTF). To eliminate distortion of the data by stimulus-onset effects, the analyses excluded the first cycle in each repetition of both components of the SAM signals.
The profile of an eMTF was used to characterize the neuronal response as low-pass, high-pass, band-pass, or band-reject (notched). Notched eMTFs were defined as profiles in which the response to one or more successive modulation frequencies was flanked by larger responses to lower and higher modulation frequencies (see Fig. 3A Lower). The best modulation frequency (BMF) at a recording site was defined as the modulation frequency that evoked the maximum entrained response. Neurons were included in this analysis only if their response at the BMF was significantly phase-locked (Rayleigh test, P ≤ 0.01; ref. 35). Statistical comparisons were based on Student's t, χ2, and Mann–Whitney U tests.
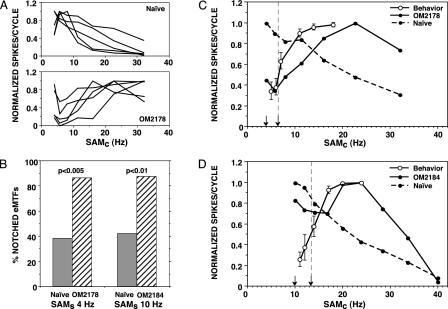
Fig. 3.
Single and compound eMTFs (spikes per cycle) normalized to maximum firing rate. (A) Examples of eMTFs recorded at five sites in naïve monkeys (Upper) and five sites in OM2178 (Lower). (B) Percentage of recording locations with notched eMTFs in trained and naïve monkeys. (C and D) Compound eMTFs for trained (solid curves) and naïve monkeys (dashed curves). p(HIT) functions (open symbols) and arrows are reproduced from Fig. 1.
To estimate neural population responses to the 1-kHz SAM signals, compound pMTFs, eMTFs, and poststimulus time histograms (PSTHs; spikes per bin) were generated for the neuronal samples obtained from trained or naïve monkeys. There were no systematic differences in the data obtained from the naïve monkeys, so the data for these animals were pooled. The number of recording sites in each sample is shown above the histograms in Fig. 1 A–D. Compound PSTHs and pMTFs were normalized for numbers of recording sites, and compound eMTFs were normalized to their maximum firing rates.
Results
Behavior. In Fig. 1 Left, psychometric curves for the two trained monkeys show the mean p(HIT) and the average RT as functions of the comparison modulation frequencies. As the frequency of SAMc increases, p(HIT) increases, RT decreases, and variability (SD bars) decreases for both of the behavioral measures.
Monkey OM2178 (Fig. 1 Left Upper) initiated 570 ± 76.4 SD trials per session (FA rate: 17.6 ± 3%). This animal's threshold and difference limen (DL) were 6.4 and 2.4 Hz, respectively. Monkey OM2184 (Fig. 1 Left Lower) initiated 363 ± 81.8 SD trials per session (FA rate: 15.7 ± 6%), and the monkey's threshold and DL were 13.2 and 3.2 Hz, respectively. These temporal discrimination thresholds are similar to those reported for a 1-kHz SAM tone in macaque monkeys (36), but they are higher than human thresholds for low-frequency SAM tonal carriers (37, 38).
Population Temporal Response Profiles of AI Neurons. In Fig. 1 the temporal profiles of neuronal responses recorded in trained and naïve monkeys are displayed in compound PSTHs normalized for the number of recording sites. The standard components (0–500 ms) are set at the modulation frequencies used in psychophysical training in monkey OM2178 (Fig. 1 A; SAMs = 4 Hz) and monkey OM2184 (Fig. 1B; SAMs = 10 Hz). Modulation frequencies of four comparison components (SAMc; 500–1,000 ms) are shown to the right of each PSTH. Fig. 1 C and D shows compound PSTHs for neuronal responses recorded in naïve monkeys with identical stimulus parameters.
The population data shown in Fig. 1 indicate that the sinusoidally modulated 1-kHz tone is represented in AI neurons by periodic responses that are synchronized with the envelope frequencies of the SAM signals. Fig. 1 also clearly illustrates two differences in the temporal response of neurons recorded in trained versus naïve monkeys (A vs. C and B vs. D). First, the responses evoked by SAMs frequencies are weak in the trained monkeys compared with the responses recorded in the naïve monkeys. Second, although the responses driven by SAMc frequencies in the trained animals are weaker than those recorded in naïve animals, the relative difference in the responses to lower-frequency versus higher-frequency SAMc signals is larger in trained monkeys.
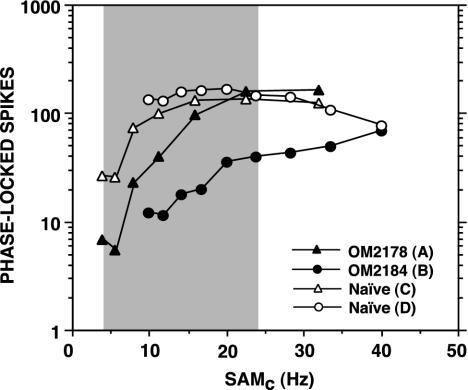
A quantitative analysis of these effects is summarized in Fig. 2, which shows compound pMTFs for the neuronal data included in Fig. 1. The pMTFs are based on the total number of phase-locked spikes (P < 0.001) evoked at each SAM frequency, normalized for number of recording sites. The shaded region in Fig. 2 indicates the overall range of SAM frequencies used in behavioral training; the key corresponds to Fig. 1 A–D.AtSAMs frequencies (4 or 10 Hz) and at 9 of the 10 signals that fall within the range of SAMc frequencies used in behavioral training, the trained monkeys' pMTFs (Fig. 2, filled symbols) are attenuated or suppressed compared with the compound phase-locked responses recorded in the naïve monkeys (Fig. 2, open symbols). However, the relative increase in phase-locked discharges is greater in the trained animals (Fig. 2, A vs. C and B vs. D). Similar results were obtained for an analysis based on mean spike rate (data not shown).
Fig. 2.
Compound pMTFs (number of phase-locked spikes) normalized for the number of recording sites. pMTFs are based on the neuronal data in Fig. 1. The key corresponds to A–D in Fig. 1. The shaded area depicts the range of SAMc frequencies used in behavioral training.
A statistical analysis of the mean number of phase-locked spikes per recording location showed that the magnitude of the responses to the SAMc frequencies just above and just below the monkeys' psychophysical thresholds were significantly different (OM2178: 6 vs. 8 Hz, t = 3.578, df = 23, P < 0.002; OM2184: 12 vs. 14 Hz, t = 2.457, df = 18, P < 0.025), but there was no difference in response magnitude between SAMs frequencies and SAMc frequencies that were below psychophysical thresholds. Thus, the number of phase-locked spikes provided a potential cue for discrimination of the SAM signals.
Comparison of Temporal Information Processing in the AI with Behavior. Entrainment (driven spikes per cycle) was chosen as the preferred neural metric for comparison of temporal coding in AI neurons with the psychophysical results, because entrainment is a measure of the response per modulation period rather than per arbitrary stimulus duration. The analysis produced a surprising result. Most of the eMTFs for the neurons recorded in the trained monkeys were notched (compare Methods). For the neurons recorded in naïve monkeys, however, most of the eMTFs were low-pass or band-pass. Representative examples of notched eMTFs recorded in OM2178 and low-pass or band-pass eMTFs recorded in naïve monkeys are shown in Fig. 3A. The percentages of eMTFs in which a notch occurred at one or more of the three comparison modulation frequencies closest to SAMs are compared in Fig. 3B. The differences in the proportions of notched functions in the trained and the naïve monkeys are significant (OM2178: χ2 = 8.748, df = 1, P < 0.005, one-tail; OM2184: χ2 = 5.845, df = 1, P < 0.01, one-tail).
To compare the neuronal population data with the psychophysical results, compound eMTFs for the neurons included in Fig. 2 and essential behavioral coordinates obtained from Fig. 1 Left were plotted as functions of SAMc. The neuronal-behavioral comparisons are shown in Fig. 3 C and D. In each plot the compound eMTFs are normalized to maximum firing rate, the curves with open symbols are p(HIT) functions, and the short and dashed arrows depict the behavioral SAMs and 50% detection threshold, respectively.
The most salient feature in Fig. 3 C and D is the very different profiles of the population eMTFs for neurons recorded in trained monkeys compared with neurons recorded in naïve monkeys. The eMTFs for the naïve monkey neuronal population are low-pass functions. This is not unusual, because entrainment MTF profiles in the AI are typically low-pass for repetitive low-frequency acoustic stimuli (11, 39, 40). However, the population eMTFs for the neurons recorded in trained monkeys are very unusual. Within the behavioral range of the SAM signals, the population functions have nonmonotonic band-reject or notched profiles that reflect the preponderance of notched neuronal eMTFs. The maximum entrained responses in the trained animals occur at SAMc frequencies that are more than twice as high as corresponding frequencies in the naïve animals. Hence in the trained monkeys, the maximum entrained population responses are located in the neighborhood of the SAMc frequencies that were frequently paired with reward during behavioral training. Interestingly, the differences in the number of entrained spikes to SAMs frequencies and SAMc frequencies in the neighborhood of the psychophysical thresholds were not significant. Threshold discrimination of a change in the modulation frequency of the 1-kHz tone appears to have occurred when the number of entrained spikes was near a minimum (Fig. 3 C and D) and when the number of phase-locked spikes to SAMc frequencies increased significantly compared with the response to SAMs frequencies (Fig. 2).
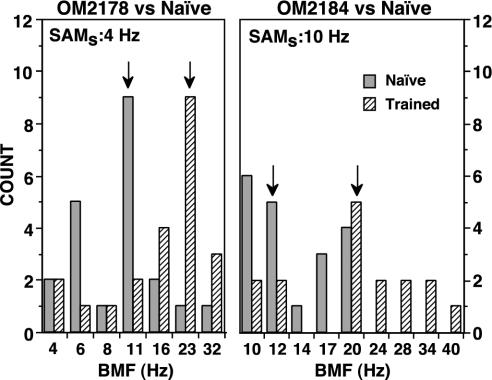
BMF. The frequency histograms in Fig. 4 compare the distributions of entrainment BMFs recorded in the trained monkeys with those recorded in the naïve monkeys. In OM2178 vs. naïve (Fig. 4 Left) and OM2184 vs. naïve (Fig. 4 Right), the histograms for the BMFs recorded in trained monkeys are shifted toward higher modulation frequencies. The large differences in median BMFs (arrows) are significant (11.3 Hz, U = 114, z = –2.895, P < 0.004 and 8.1 Hz, U = 63, z = –3.004, P < 0.003, respectively).
Fig. 4.
Frequency histograms of BMFs for neurons recorded in naïve and trained monkeys. Arrows indicate median BMFs.
Discussion
Training on a temporal discrimination task had two important effects on the response of AI neurons, and both reflect an encoding strategy that preserved the temporal correlation between the cadences of the stimulus and the neuronal response while enhancing the representation in the AI of higher-frequency SAM signals that were frequently paired with reward.
First, cortical responses to SAM signals were globally suppressed or inhibited in the trained monkeys, whereas identical stimuli were represented by vigorous AI neuronal responses in the naïve animals. The suppressive effects observed in the trained animals were expressed in both the representational pattern and magnitude of AI responses. Although the number of phase-locked spikes was reduced in the trained monkeys, suppression actually improved the contrast between responses to lower-frequency versus higher-frequency SAM signals. Compared with behavioral subthreshold SAM frequencies, the number of phase-locked spikes increased significantly at suprathreshold SAM frequencies, providing a potential temporal cue for threshold discrimination of a change in the cadence of the sinusoidally modulated 1-kHz tone.
Second, a localized suppression of entrained responses that was concentrated in the neighborhood of the lower-frequency SAM signals significantly increased the proportion of notched eMTFs in the trained animals (cf. also ref. 21). As a result of this local effect, the normalized population entrainment functions in the trained monkeys were characterized by nonmonotonic, notched profiles with maximum entrained responses to the higher SAM frequencies presented during behavioral training. By contrast, the population eMTFs observed in the naïve animals had low-pass profiles, i.e., the magnitude of the entrained response varied inversely with the frequency of SAM. This remarkable difference in the relative effectiveness of higher SAM frequencies in trained and naïve animals produced a shift in the distribution of BMFs in the trained monkeys that significantly increased the proportion of neurons that “preferred” higher modulation frequencies.
These effects imply behaviorally contingent inhibitory and excitatory processes that are modulated by the probability that a particular signal predicts a reward. Nonreinforced and occasionally reinforced signals were represented weakly in the AI; consistently reinforced signals had a relatively robust representation in the AI. In several respects, these effects have features that are similar to phenomena reported in learning studies that use classical (Pavlovian) conditioning procedures. In both cases, responses reflect both inhibitory and excitatory plasticity effects.
Modern Pavlovian conditioning theory emphasizes the importance of the contingency or correlation between a conditioned stimulus (CS) and an unconditioned stimulus (US) for elaboration of a conditioned response (CR) (31, 32). According to the rules of Pavlovian conditioning, if a CS is consistently paired with reward (US), the CS acquires excitatory properties (CS+) that enable it to evoke a CR. Conversely, if a stimulus that is never paired with reward is included in the conditioning procedure, it acquires inhibitory properties (CS–). If the CS– is presented alone, the CR will be actively inhibited; in the presence of a compound stimulus, CS+/CS–, the magnitude of the CR will be reduced (27, 28, 30). These basic features of Pavlovian conditioning suggest that excitatory conditioning involves the formation of associations between central representations of the CS and the US, whereas inhibitory conditioning involves the formation of associations between internal representations of the CS and events that occur when the US is omitted (25). Because the prerequisites for Pavlovian conditioning exist implicitly in the context of any instrumental learning task, contemporary learning theory assumes that Pavlovian inhibitory and excitatory conditioning processes modulate instrumental learning and performance by the formation of associations between stimuli (25, 26, 29, 30).
We propose that the cortical plasticity observed in the present study is a residue of Pavlovian inhibitory and excitatory conditioning processes that modulated the responses of auditory neurons during instrumental learning and performance of a temporal discrimination task, i.e., the responses recorded in populations of AI neurons in the trained monkeys are neuronal representations of the SAM signals used in training, modulated in accordance with Pavlovian rules of conditioning. To evaluate this hypothesis, we refer to Table 1, which shows the kinds of trials, the stimulus temporal orders, the equivalent Pavlovian contingencies, and the hypothetical neural effects that occurred during training.
Table 1. Kind of trial, stimulus temporal order, Pavlovian stimulus equivalent, and effect on AI neural discharge.
| Trial | Stimulus temporal order/Pavlovian stimulus equivalent | Effect on AI neural discharge |
|---|---|---|
| FA | SAMs — TO (CS- — CS-) | Inhibition |
| Hit | SAMs — SAMc — R (CS- — CS+ — US) | Excitation |
| Miss | SAMs — SAMc — TO (CS- — CS- — CS-) | Inhibition |
| Backward conditioning | R — SAMs (US — CS-) | Inhibition |
Bold type indicates inhibitory stimuli. R, reward.
FA trials were frequent during the earlier stages of learning and occurred on approximately one-sixth of trials after a monkey's performance had stabilized. The anticipatory decision to respond always happened during SAMs, and every FA was followed by a TO that delayed the opportunity for reward. In the context of the conditioning stimulus events that occurred in training, SAMs signals and TOs are both inhibitory CSs. The stimulus sequence SAMs/TO, or its Pavlovian equivalent, CS–/CS–, is therefore a compound inhibitory CS associated with the omission of a reward (US), and this purely inhibitory CS actively suppressed AI neuronal responses to the SAMs signals.
Hit trials occurred whenever a monkey responded appropriately to a change in the modulation frequency of the SAM signals. Although hits were always rewarded, the probability of a hit covaried directly with the magnitude of the difference between the SAMs and SAMc frequencies. Notably, on hit trials the stimulus contingency included a compound CS with an inhibitory component preceding the excitatory component (SAMs/SAMc or CS–/CS+). The effect of the inhibitory component was to suppress the strength of AI neuronal responses differentially. Representations of lower-frequency, partially reinforced SAMc signals were inhibited, whereas representations of higher-frequency, reinforced SAMc signals were relatively uninhibited. Because the magnitude of the suppression tended to covary with the probability that a signal was rewarded during training, the stimulus contingency on hit trials produced a form of differentially conditioned inhibition (30). The observed effects of this contingency are consistent with evidence indicating that the correlation with reinforcement is a critical variable in Pavlovian conditioning (25).
Miss trials occurred whenever a monkey failed to respond appropriately to a change in the modulation frequency of the SAM signals, and, in exact opposition to hit trials, the probability of a miss covaried directly with the similarity between the SAMs and SAMc frequencies. The stimulus contingency on a miss trial consisted of three inhibitory components: SAMs, an ineffective or subthreshold SAMc and a TO. Because this compound inhibitory CS was associated with the omission of reward (US), its inhibitory effect was potentially very strong and probably contributed to the severe suppression of cortical responses at lower-frequency SAMc signals where the miss rate was ≈25–75%.
The least obvious kind of trial is backward conditioning, wherein the usual CS/US pairing is reversed: US/CS. After every US (reward), a CS (SAMs) was delivered immediately when a monkey initiated the next trial. The two stimuli were strongly correlated on hit trials, but the contingency was a negative one: SAMs predicted a period in which a reward would not occur (32). In his seminal study of nervous system plasticity, Konorski (27) considered backward conditioning to be the most effective conditioning procedure to produce inhibition, and this contingency probably contributed to the profound suppression of AI neuronal responses to SAMs signals and to SAMc signals that were indistinguishable from the standard signals.
Conclusion
We presume that the cortical plasticity observed in the present study was induced in accordance with Pavlovian rules of conditioning that modulated the responses of auditory neurons during instrumental learning and performance of a temporal discrimination task. Training produced inhibitory conditioning on virtually every trial. The presentation of a nonreinforced standard signal at the beginning of each trial, the influence of backward conditioning, and the TO delay of reinforcement on FA and miss trials provided a powerful inhibitory context that affected neuronal responses to each of the stimulus contingencies included in Table 1. The overall effect was global and localized suppression of the representation of SAM signals in AI that was partially offset by phase-locked responses that were strongly entrained to frequently rewarded signals.
Three limitations of the present study should be noted. First, the physiological results were obtained from anesthetized animals, and although plasticity is expressed under anesthesia in sensory neocortex (41, 42), anesthetics can potentiate the effects of inhibition (43, 44). Second, inhibitory influences arise from local neuronal networks in the auditory cortex and from inhibitory interactions projected from lower levels of the auditory system (45–48), so we cannot exclude the possibility that the results depended on subcortical sources of inhibition. Finally, the experimental design of our study precluded investigation of either the neuromodulatory systems that are implicated in neuronal response suppression and enhancement within various behavioral contexts (for reviews, see refs. 19 and 49) or the putative physiological mechanisms involved in inhibitory neuronal plasticity (27).
Acknowledgments
Drs. D. Blake and D. Polley made helpful comments on the manuscript. This research was supported by a Department of Veterans Affairs Merit Review grant (to S.W.C.), National Institutes of Health Grants NS-10414 and NS-34835, and Coleman Fund and Hearing Research, Inc.
Abbreviations: AI, primary auditory cortex; SAM, sinusoidally amplitude modulated; RT, reaction time; FA, false alarm; TO, timeout; p(HIT), proportion of correct responses; CF, characteristic frequency; MTF, modulation transfer function; pMTF, phase-locked MTF; eMTF, entrained MTF; BMF, best modulation frequency; PSTH, poststimulus time histogram; CS, conditioned stimulus; US, unconditioned stimulus.
References
- 1.Pelleg-Toiba, R. & Wollberg, Z. (1991) J. Basic Clin. Physiol. Pharmacol. 2, 257–272. [DOI] [PubMed] [Google Scholar]
- 2.Steinschneider, M., Arezzo, J. C. & Vaughan, H. G., Jr. (1982) Brain Res. 252, 353–365. [DOI] [PubMed] [Google Scholar]
- 3.Wang, X., Merzenich, M. M., Beitel, R. & Schreiner, C. E. (1995) J. Neurophysiol. 74, 2685–2706. [DOI] [PubMed] [Google Scholar]
- 4.Benson, D. F. & Geschwind, N. (1969) in Handbook of Clinical Neurology, eds. Vinken, P. J. & Bruyn, G. W. (North–Holland, Amsterdam), Vol. 4, pp. 112–140. [Google Scholar]
- 5.Heffner, H. & Heffner, R. (1986) J. Neurophysiol. 56, 683–701. [DOI] [PubMed] [Google Scholar]
- 6.Andrew, R. J. (1963) Behaviour 20, 1–109. [Google Scholar]
- 7.Moynihan, M. (1964) Smithsonian Misc. Collect. 146, 1–84. [Google Scholar]
- 8.Rosen, S. (1992) Philos. Trans. Soc. London B 336, 367–373. [DOI] [PubMed] [Google Scholar]
- 9.Bieser, A. & Muller-Preuss, P. (1996) Exp. Brain Res. 108, 273–284. [DOI] [PubMed] [Google Scholar]
- 10.Eggermont, J. J. (1994) Hear. Res. 74, 51–66. [DOI] [PubMed] [Google Scholar]
- 11.Kilgard, M. P. & Merzenich, M. M. (1999) Hear. Res. 134, 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang, L., Lu, T. & Wang X. (2002) J. Neurophysiol. 87, 2237–2261. [DOI] [PubMed] [Google Scholar]
- 13.Nagarajan, S. S., Cheung, S. W., Bedenbough, P., Beitel, R. E., Schreiner, C. E. & Merzenich, M. M. (2002) J. Neurophysiol. 87, 1723–1737. [DOI] [PubMed] [Google Scholar]
- 14.Schreiner, C. E. & Urbas, J. V. (1986) Hear. Res. 21, 227–241. [DOI] [PubMed] [Google Scholar]
- 15.Schulze, H. & Langner, G. (1997) J. Comp. Physiol. A 181, 651–663. [DOI] [PubMed] [Google Scholar]
- 16.Drullman, R. (1995) J. Acoust. Soc. Am. 97, 585–592. [DOI] [PubMed] [Google Scholar]
- 17.Shannon, R. V., Zeng, F.-G., Kamath, V., Wygonski, J. & Ekelid, M. (1995) Science 270, 303–304. [DOI] [PubMed] [Google Scholar]
- 18.Buonomano, D. V. & Merzenich, M. M. (1998) Annu. Rev. Neurosci. 21, 149–186. [DOI] [PubMed] [Google Scholar]
- 19.Edeline, J.-M. (1999) Prog. Neurobiol. 57, 165–224. [DOI] [PubMed] [Google Scholar]
- 20.Ohl, F. W. & Scheich, H. (1996) Eur. J. Neurosci. 8, 1001–1017. [DOI] [PubMed] [Google Scholar]
- 21.Ohl, F. W. & Scheich, H. (1997) J. Comp. Physiol. A 181, 685–696. [DOI] [PubMed] [Google Scholar]
- 22.Scheich, H. (1991) Curr. Opin. Neurobiol. 1, 236–247. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger, N. M. (1993) Curr. Opin. Neurobiol. 3, 570–577. [DOI] [PubMed] [Google Scholar]
- 24.Blake, D. T., Strata, F., Churchland, A. K. & Merzenich, M. M. (2002) Proc. Natl. Acad. Sci. USA 99, 10114–10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickinson, A. & Mackintosh, N. J. (1978) Annu. Rev. Psychol. 29, 587–612. [DOI] [PubMed] [Google Scholar]
- 26.Hearst, E. (1988) in Stevens' Handbook of Experimental Psychology, eds. Atkinson, R. C., Herrnstein, R. J., Lindzey, G. & Luce, R. D. (Wiley, New York), Vol. 2, pp. 3–109. [Google Scholar]
- 27.Konorski, J. (1948) Conditioned Reflexes and Neuron Organization (Cambridge Univ. Press, Cambridge, U.K.).
- 28.Pavlov, I. P. (1927) Conditioned Reflexes (Oxford Univ. Press, London).
- 29.Pearce, J. M. & Hall, G. (1980) Psychol. Rev. 87, 532–552. [PubMed] [Google Scholar]
- 30.Rescorla, R. A. & Soloman, R. L. (1967) Psychol. Rev. 74, 151–182. [DOI] [PubMed] [Google Scholar]
- 31.Rescorla, R. A. (1967) Psychol. Rev. 74, 71–80. [DOI] [PubMed] [Google Scholar]
- 32.Rescorla, R. A. (1988) Annu. Rev. Neurosci. 11, 329–352. [DOI] [PubMed] [Google Scholar]
- 33.Imig, T., Ruggero, M., Kitzes, L., Javel, E. & Brugge, J. (1977) J. Comp. Neurol. 171, 111–128. [DOI] [PubMed] [Google Scholar]
- 34.Recanzone, G. H., Schreiner, C. E., Sutter, M. L., Beitel, R. E. & Merzenich, M. M. (1999) J. Comp. Neurol. 415, 460–481. [DOI] [PubMed] [Google Scholar]
- 35.Mardia, K. V. (1972) Statistics of Directional Data (Academic, London).
- 36.Moody, D. B. (1994) J. Acoust. Soc. Am. 95, 3499–3510. [DOI] [PubMed] [Google Scholar]
- 37.Formby, C., Morgan, L. N., Forrest, T. G. & Raney, J. J. (1992) J. Acoust. Soc. Am. 91, 293–305. [DOI] [PubMed] [Google Scholar]
- 38.Lee, J. (1994) J. Acoust. Soc. Am. 96, 2140–2147. [DOI] [PubMed] [Google Scholar]
- 39.Eggermont, J. J. (1991) Hear. Res. 56, 153–167. [DOI] [PubMed] [Google Scholar]
- 40.Phillips, D. P., Hall, S. E. & Hollet, J. L. (1989) J. Acoust. Soc. Am. 85, 2537–2549. [DOI] [PubMed] [Google Scholar]
- 41.Recanzone, G. H., Schreiner, C. E. & Merzenich, M. M. (1993) J. Neurosci. 13, 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinberger, N. M., Javid, R. & Lepan, B. (1993) Proc. Natl. Acad. Sci. USA 90, 2394–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franks, N. P. & Lieb, W. R. (1994) Nature 367, 607–614. [DOI] [PubMed] [Google Scholar]
- 44.Zurita, P., Villa, A. E. P., de Ribaupierre, Y., de Ribaupierre, F. & Rouiller, E. M. (1994) Neurosci. Res. 19, 303–316. [DOI] [PubMed] [Google Scholar]
- 45.Phillips, D. P. (1988) J. Neurophysiol. 59, 1524–1539. [DOI] [PubMed] [Google Scholar]
- 46.Schreiner, C. E. & Mendelson, J. R. (1990) J. Neurophysiol. 64, 1442–1459. [DOI] [PubMed] [Google Scholar]
- 47.Shamma, S. A. & Symmes, D. (1985) Hear. Res. 19, 1–13. [DOI] [PubMed] [Google Scholar]
- 48.Sutter, M. L., Schreiner, C. E., McLean, M., O'Conner, K. N. & Loftus, W. C. (1999) J. Neurophysiol. 82, 2358–2371. [DOI] [PubMed] [Google Scholar]
- 49.Schultz, W. & Dickinson, A. (2000) Annu. Rev. Neurosci. 23, 473–500. [DOI] [PubMed] [Google Scholar]