Abstract
At the first stage of processing in the olfactory pathway, the patterns of glomerular activity evoked by different scents are both temporally and spatially dynamic. In the antennal lobe (AL) of some insects, coherent firing of AL projection neurons (PNs) can be phase-locked to network oscillations, and it has been proposed that oscillatory synchronization of PN activity may encode the chemical identity of the olfactory stimulus. It remains unclear, however, how the brain uses this time-constrained mechanism to encode chemical identity when the stimulus itself is unpredictably dynamic. In the olfactory pathway of the moth Manduca sexta,we find that different odorants evoke gamma-band oscillations in the AL and the mushroom body (a higher-order network that receives input from the AL), but oscillations within or between these two processing stages are not temporally coherent. Moreover, the timing of action potential firing in PNs is not phase-locked to oscillations in either the AL or mushroom body, and the correlation between PN synchrony and field oscillations remains low before, during, and after olfactory stimulation. These results demonstrate that olfactory circuits in the moth are specialized to preserve time-varying signals in the insect's olfactory space, and that stimulus dynamics rather than intrinsic oscillations modulate the uniquely coordinated pattern of PN synchronization evoked by each olfactory stimulus. We propose that non-oscillatory synchronization provides an adaptive mechanism by which PN ensembles can encode stimulus identity while concurrently monitoring the unpredictable dynamics in the olfactory signal that typically occur under natural stimulus conditions.
In the olfactory systems of widely divergent species, the patterns of odor-evoked neural activity that spread across the olfactory glomeruli at the first stage of processing are both spatially and temporally complex (1–7). The distributed and dynamic nature of these central responses has also complicated efforts to decipher the rules underlying olfactory stimulus identification and discrimination in the brain. In the antennal lobe (AL) of insects, the equivalent of the olfactory bulb in vertebrates, increasing evidence suggests that specific patterns of synchronous firing across a distributed array of AL projection neurons (PNs) may encode different features of an olfactory stimulus (8–12). For example, some studies suggest that global network oscillations can be used as a periodic time reference to help coordinate and update (with each oscillation cycle) the specific PN ensemble that encodes the chemical identity of the olfactory stimulus (3, 8). It remains unresolved, however, whether a temporally structured mechanism like oscillatory synchronization can reliably encode information about the chemical identity of a stimulus that is itself temporally unpredictable. We used the sphinx moth Manduca sexta, an animal that possesses anatomically discrete and identifiable glomeruli and shows robust behavioral responses to different olfactory stimuli (4, 9–12), to test for a functional relation between network oscillations and the temporal pattern of PN firing evoked by olfactory stimuli that target specific glomeruli. We used simultaneous single-unit or ensemble recordings (12) coupled with local field potential (LFP) (13, 14) recordings to investigate the temporal relationships between PN spike activity and the macroscopic oscillations evoked by both pheromonal and non-pheromonal stimuli. Our results show that although the moth olfactory system clearly exhibits odor-evoked LFP oscillations (14), the stimulus-specific coordination of PN ensembles is not oscillatory, but modulated transiently by the dynamics of the olfactory stimulus itself.
Experimental Procedures
Laboratory-reared Manduca sexta males were prepared for electrophysiological recordings as described (9, 14). Glass electrodes for intracellular recording had tip resistances of 100–400 MΩ when filled with 3% Lucifer Yellow dye; those for LFP recordings measured <10 MΩ when filled with 2 M LiCl. Both the intracellular and LFP signals were acquired and digitized at a sampling rate of 25 kHz per channel. During data acquisition, the LFP signal was low-pass filtered at 100 Hz. Intracellular electrodes were always placed directly into the macroglomerular complex (MGC), whereas the LFP electrodes were inserted at different locations in the AL, depending on whether we wanted to compare the responses within or between AL glomeruli. To examine the temporal relationship between the odor-evoked activity patterns across key brain structures in the insect olfactory pathway, one LFP electrode was inserted into the AL and the other into the calyces of the ipsilateral mushroom body in the protocerebrum. To examine population responses to olfactory stimulation, neural-ensemble recordings were obtained with silicon-based multielectrode arrays (Center for Neural Communication Technology, University of Michigan, Ann Arbor) feeding into twin 8-channel Neurolynx amplifiers as reported (ref. 12; see Supporting Methods, which is published as supporting information on the PNAS web site, www.pnas.org).
Results
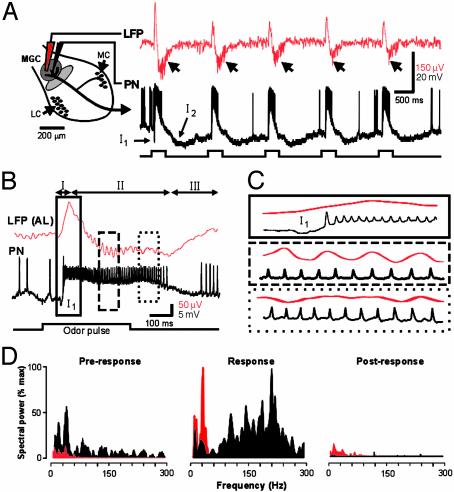
Physiological Properties of Moth PNs. In the insect AL, PN dendrites receive synaptic input exclusively within the olfactory glomeruli and then relay processed information to several sites in the protocerebrum, including the mushroom body (MB) (15, 16). We used similar recording methods to those in earlier studies (3, 8, 14, 17) to analyze the temporal relationships between PN spikes and LFP oscillations at these two processing stages in the moth olfactory pathway. An example of simultaneous intracellular and surface LFP recordings from the same glomerular region of the AL (the male MGC) is shown in Fig. 1. The physiological characteristics of PNs in both male and female moths are distinctly different from those reported in some other insects. For example, PNs in male and female moths do not exhibit slow patterning in their responses to brief olfactory stimuli (3, 8, 17). Rather, PN responses in the moth are multiphasic (not unlike those observed in some vertebrate mitral/tufted cells; ref. 4), are locked to stimulus onset, and typically do not outlast the stimulus (Fig. 1 A). Similarly, the LFP response in the AL was also multiphasic (14) (Fig. 1B). An early surface-positive component (phase I) preceded an equally rapid negative component (phase II) followed by a gradual recovery to prestimulus levels (phase III) (Fig. 1B). Phase I had a smaller peak-to-peak amplitude than phase II (386.3 ± 271.2 μV vs. 614.8 ± 471.1 μV; n = 258 responses in 14 moths), and the onset of phase I was correlated to the onset of the fast γ-aminobutyric acid (GABA)ergic, bicuculline-sensitive inhibitory postsynaptic potential (IPSP) (I1) in the PN (11). PNs began to fire during the rise in phase I, and they achieved and maintained maximum firing rates well before LFP oscillations appeared in phase II (Fig. 1C). Hence, in response to an olfactory stimulus, the bulk of the evoked PN-spike activity occurred during the nonoscillatory phases of the LFP recorded in the same glomerulus.
Fig. 1.
Simultaneous intracellular and field potential recordings illustrate that spiking activity in glomerular PNs (black) is not dependent on LFP oscillations (red). (A) Schematic drawing of the moth AL (Left) showing the approximate placement of the extracellular (LFP) and intracellular (PN) electrodes in the MGC. (Right) LFP and PN responses to five consecutive pulses of the pheromone blend (10 ng of BAL plus 10 ng of C15) are shown (stimulus duration = 500 ms; interval = 2 s). Note how both the LFP and PN responses are closely time-locked to each stimulus pulse. In the PN, this is facilitated by the large but brief IPSP (I1). Oscillations in the LFP appear only late in the response (arrowheads). (B) In another experiment, an expanded view of the first response epoch shows the details of the temporal relationship between the multiphasic LFP (phases I–III) and the PN spike train. PN spiking activity is initiated during phase I (solid outline), but LFP oscillations do not appear until phase II (dashed outline). Moreover, PN spikes are not phase-locked to the LFP at any time during the olfactory response. (C) Expanded view of boxed areas outlined in B.(D) Frequency spectra (smoothed with a 5-ms Gaussian function and normalized to maximum) computed for the PN (black) and LFP (red) before (Pre-response), during (Response), and after (Post-response) the olfactory response. Note that, during the odor-evoked response, the peak frequencies derived from the two recorded signals do not match.
LFP Oscillations Occur Spontaneously. In the moth AL, LFP oscillations (frequency range: 9.7–46.9 Hz; n = 14 moths) also occurred before the olfactory stimulus was presented, which reflects the situation in other vertebrates and invertebrates (1, 18, 19). Fourier analysis of the odor-evoked LFP responses revealed a small but significant increase in the peak frequency of the oscillation during the olfactory response (Fig. 1D). The average dominant frequency before the response was 22.8 ± 6.1 Hz (mean ± SD), whereas that during the response was 25.6 ± 7.7 Hz (Wilcoxon rank sum test, P < 0.01). After olfactory stimulation (Fig. 1D Right), the peak LFP frequency returned to its prestimulus value (22.9 ± 5.3 Hz). Spectral power at the dominant frequency also increased an average of 7.2 times during the olfactory response (Fig. 1D Center, but power always returned to prestimulus levels after the response. In sharp contrast to the LFP, most of the spectral power in the PN response shifted to much higher frequencies (in the 150- to 300-Hz range) during stimulus presentation (black trace in Fig. 1D Center). Spectral analysis therefore shows that LFP oscillations occur spontaneously, but that an olfactory stimulus greatly increases the spectral power in the resting frequency range with only a small change in the dominant frequency. Furthermore, unlike findings in other studies (3, 8, 17), in no case during olfactory stimulation did spike trains in the PN and oscillations in the LFP share the same dominant frequency.
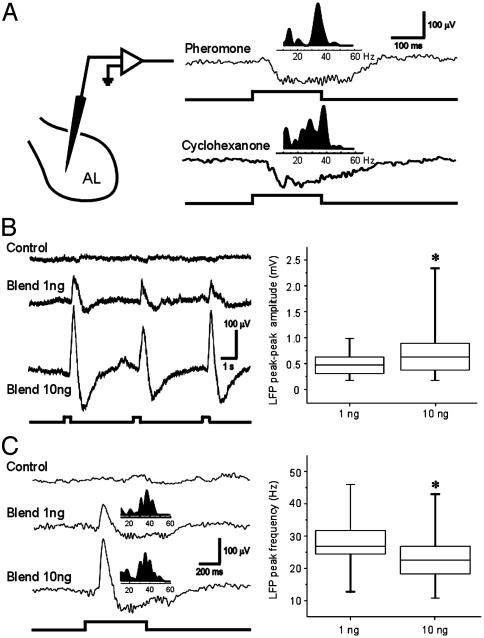
Different Odors Evoke Similar LFPs at the Same Recording Site. Do LFPs recorded in the AL exhibit any features that could be considered stimulus specific? Fourier analysis of the LFPs evoked by two chemically different stimuli often revealed different spectral peaks in the gamma band. In the example in Fig. 2A, LFP oscillations evoked by stimulation with the sexpheromone blend showed a spectral peak at 35 Hz, whereas the maximum power evoked by a simple ketone, cyclohexanone, occurred at 38 Hz when recorded at the same site. When we analyzed these results across animals, however, we found that the spectral peaks evoked by pheromone and cyclohexanone were not significantly different (23.3 ± 8.7 vs. 27.1 ± 9.7 Hz; P = 0.146). We also found no significant difference in the spectral peaks evoked by these two stimuli within a single preparation (mean P value = 0.182 ± 0.16). When recorded at the same site, widely different odorants therefore evoke similar LFP responses in the gamma frequency range. This finding is generally consistent with results in other olfactory systems, including studies in both invertebrates and vertebrates that reported only small changes in the frequency of the LFP oscillation with different olfactory stimuli (1, 6). Importantly, however, in this and other studies that test only a limited number of olfactory stimuli, it is impossible to say (based on currently available methods) whether any of these neural patterns are stimulus specific.
Fig. 2.
Effects of odor chemistry and stimulus intensity on evoked LFPs. (A) LFP recorded near the center of the AL (Left). Raw LFP recordings (Right) show that different olfactory stimuli evoke similar temporal patterns in the LFP. Fourier analysis of the two responses (Insets) also reveals similar spectral peaks of 34 and 38 Hz for the pheromone blend (10 ng of BAL plus 10 ng of C15) and cyclohexanone (10 μg), respectively. (B) Effect of stimulus intensity on the LFP response to pheromone. Raw LFP traces (Left) reveal a dose-dependent effect on response amplitude along with a small reduction in peak spectral frequency (36 Hz for 1 ng of blend; 34 Hz for 10 ng of blend). Box plots based on pooled data from 35 trials (Right) illustrate the significant effect of stimulus intensity on peak-to-peak LFP amplitude (Wilcoxon rank sum test, P < 0.01). Boxes mark the upper and lower quartiles, the line inside each box marks the sample median, and vertical bars show the sample range. (C) Recordings from a different preparation illustrate the spectral changes in the LFP responses triggered by different stimulus intensities. Frequency analysis (Insets above raw LFP traces) reveals a spectral peak at 38 Hz for the 1-ng blend, but a reduction to 34 Hz for the 10-ng blend. (Right) Box plots based on pooled data (n = 35 trials) confirm a small but significant decrease in the mean LFP peak frequency with increasing stimulus intensity (P < 0.01).
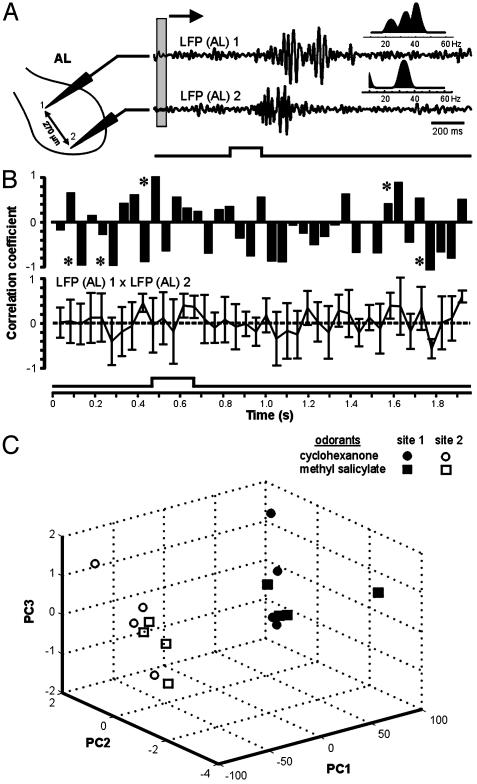
Could the subtle differences in peak LFP frequency be explained by a functional disparity between the pheromonal and nonpheromonal systems in the moth AL? To test this possibility, we also compared the LFP responses evoked by two nonpheromonal odorants (cyclohexanone and methyl salicylate) recorded simultaneously at two separate sites outside of the MGC in the AL (Fig. 3A). Principal components analysis revealed a striking separation between the clusters of LFP responses recorded at different sites, whereas the responses to the two stimuli recorded at the same site showed much less variation (Fig. 3C). These data suggest that LFP responses may not propagate globally, but are spatially restricted to different regions of the AL, perhaps to different glomeruli (see below).
Fig. 3.
LFPs evoked by the same olfactory stimulus but recorded from spatially distinct sites in the same neuropil are not temporally coherent. (A) Spatial variation in stimulus-evoked LFP responses recorded simultaneously from two different glomerular sites separated by 270 μm. Cyclohexanone evokes different LFP patterns across the AL, and these patterns show different spectral properties (Insets). Gray box depicts the 50-ms sliding window used to calculate the running correlations in B. LFPs were band-passed from 5 to 55 Hz. (B) Sliding-window plots of the time course of correlation between the two LFPs reveal no evidence for global coherence before, during, or after a 200-ms odor pulse. Sliding windows of 20, 50, and 100 ms were used to calculate running correlations; all showed the same outcome; thus, only the results using the 50-ms window are shown. The upper trace shows the covariation for the first of a series of five odor pulses separated by 2 s; the lower trace shows the data averaged over the five pulses (mean ± SD). Note that the strongest correlations (asterisks) did not occur during peak LFP activity. (C) Principal components analysis (PCA) showed that, irrespective of the stimulus, LFP responses recorded at the same site showed considerably less temporal variation than those recorded at different sites. The LFP responses evoked by two nonpheromonal stimuli (cyclohexanone and methyl salicylate, four trials each) were recorded simultaneously at two separate sites outside of the MGC, as shown in A. PCA revealed a clear separation between the clusters of LFP responses recorded at the two sites (99% of the variation was associated with the first three eigenvectors in the 3D plot). The strong segregation of clusters between the two sites suggests that LFP responses may not propagate globally, but are instead localized to different regions of the AL, perhaps even to different glomeruli (see text).
Different Odor Intensities Yield Different Oscillation Frequencies. A critical issue that has received little attention is how the temporal and spectral characteristics of the LFP might be modulated by changes in stimulus intensity. To address this specific question, we recorded odor-evoked LFP activity from the male-specific MGC and tested with the sex-pheromone blend that specifically activates these glomeruli (4, 9–12). The pheromone blend was delivered as pulses at different stimulus intensities to simulate a natural wind-borne odor plume (20). As shown in Fig. 2B, both concentrations evoked a typical multiphasic LFP, but the response was strongly dose dependent. The higher dosage (10 ng) was associated with a significant increase in the peak-to-peak LFP amplitude (Fig. 2B; P < 0.01; n = 35 trials), as well as a small but significant reduction in peak oscillation frequency (P < 0.01; Fig. 2C). These results therefore indicate that both the amplitude and spectral properties of the LFP vary in a concentration-dependent manner, providing further evidence that LFP dynamics alone do not provide a reliable indicator of stimulus identity.
Are Oscillations “Globally Coherent”? Another key question that must be addressed before functional significance can be assigned to LFP oscillations is: how “local” is the LFP? Previous studies concluded that “globally coherent” oscillations in the MB reflect the distributed firing patterns of PN input from the AL (21). To test this hypothesis, we first recorded LFP responses to cyclohexanone from two sites in the AL that were separated by at least 200 μm and were therefore situated in different glomeruli (Fig. 3A). As predicted from the distributed organization of sensory input from the antenna to the array of glomeruli in the AL (10, 11), these paired recordings revealed robust stimulus-evoked LFPs at both sites. Importantly, however, the two LFP responses did not exhibit the same time course, nor were they temporally coherent (Fig. 3 A and B). This was demonstrated in two ways. First, Fourier analysis revealed that LFPs evoked by the same stimulus but recorded at different sites each exhibited unique spectral characteristics (n = 8 moths). In Fig. 3A, for example, note the contrast between the multipeaked spectrum calculated from the recording at site 1 and the single-peaked spectrum from site 2. Second, sliding-window correlation analysis revealed no difference in temporal coherence between the two LFPs recorded before, during, or after the olfactory stimulus (Fig. 3B). A global correlation between the LFPs should have resulted in one or more discrete peaks during the olfactory response (17), but no such pattern was observed. Furthermore, peaks of LFP coherence across sites did not always occur during the stimulus.
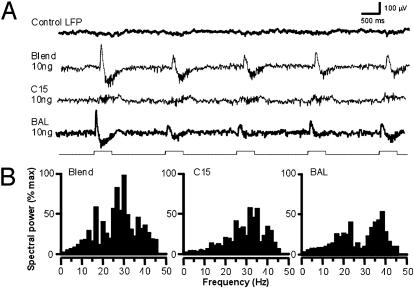
In another experiment, we took advantage of the fact that in M. sexta, processing of information about each of the two key components of the sex pheromone occurs in a different identified glomerulus in the MGC (9, 12, 14). By delivering the two stimuli separately we were able to drive input to each glomerulus independently. We recorded LFP responses from one of these glomeruli (the toroid), which receives input from afferent axons carrying information about the pheromone component bombykal (BAL). The LFP response to BAL displayed the familiar multiphasic time course (Fig. 1). However, when we used a stimulus (C15) that drives selective input to the neighboring glomerulus (the cumulus), the LFP waveform recorded in the toroid was dramatically different (Fig. 4). Olfactory input to the cumulus evoked only weak oscillations in the toroid LFP without the slower and larger positive or negative phases. This finding suggests that the strong phasic modulations of the LFP are generated only in the glomerulus that receives direct input from activated receptor cells. Input to the adjacent glomerulus (in the above example, the cumulus) does not reveal the same pattern, presumably because the local population dynamics initiated in one glomerulus are not transmitted laterally to its neighbors. As further evidence of this functional separation of field potentials between glomeruli, although the spectral properties of the LFP responses evoked by the two olfactory inputs fell within the same range (i.e., 30–40 Hz), those responses nevertheless had power maxima at different spectral peaks (Fig. 4B). Thus, we found no evidence that LFP oscillations are globally coherent potentials that can serve as a time reference for temporal coding across the AL glomeruli. In fact, our data are more consistent with the hypothesis that each glomerulus may produce its own LFP in response to selective olfactory input.
Fig. 4.
The temporal structure of the LFP depends on whether the glomerulus receives direct input from the olfactory stimulus. (A) LFP was recorded from the toroid, a large glomerulus in the male's MGC that receives specific input from one component of the female sex pheromone, BAL. The complete pheromone (Blend) and BAL evoked multiphasic LFPs, whereas C15 (a mimic of the second pheromone component that selectively stimulates the adjacent glomerulus) evoked only oscillations. (B) Power-spectral analysis performed over the five trials shows that the distribution of frequency peaks is different for BAL and C15, and different still for the blend of the two olfactory stimuli.
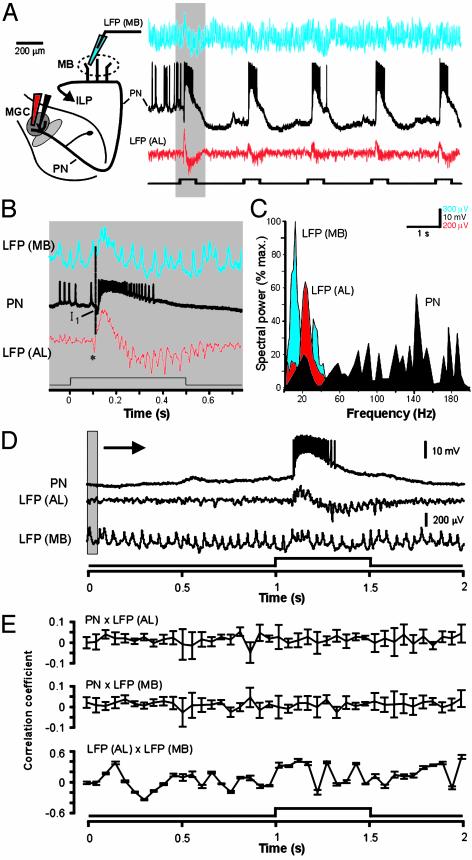
If LFPs in the AL are not globally coherent, how coherent are LFPs recorded simultaneously in the AL and the ipsilateral MB, an area that receives major projections from the AL? To address this question, we moved one LFP recording electrode to the MB and examined the temporal relationship between the patterns of odor-evoked oscillatory activity in these two processing centers (Fig. 5 A and B). Once again, Fourier analysis revealed little temporal correspondence between PN spike activity and LFPs recorded simultaneously in either the AL or the MB (Fig. 5C; n = 3 moths). We found, moreover, that the correlation between PN activity and either LFP trace did not change before, during, or after odor stimulation (Fig. 5 D and E; results with 50-ms sliding window are shown; smallest P value = 0.31). We also found only a very weak periodic relationship between LFPs recorded simultaneously in the AL and MB (P = 0.09), and the strongest correlations between these two centers occurred at random, often before stimulus onset (Fig. 5E).
Fig. 5.
Lack of coincidence structure between PN spike trains (black) recorded simultaneously with LFPs from the MB and AL. (A) Each stimulus pulse modulates network activity at both sites: LFP (MB) is shown in blue; LFP (AL) is shown in red. The stimulus protocol was five consecutive pulses of cyclohexanone, each 500 ms in duration and separated by 2 s (lower trace). (B) Expanded view of traces from shaded area in A. The downward deflection (asterisk) in LFP (AL) likely represents the synchronous arrival of excitatory afferent input to the glomerulus. (C) Spectral analysis reveals no overlap in the peak frequencies between LFPs, and no correspondence between either LFP and peak PN activity. (D) Lack of correlation between PN spiking and LFPs recorded simultaneously in the AL and MB. Raw PN and LFP traces illustrate the response to a single 500-ms pulse of sex pheromone. The shaded area depicts the sliding window (50 ms) used to calculate moving correlations in E. (E) Sliding-window correlations calculated between PN membrane potential and LFPs, measured before, during, and after olfactory stimulation; results using the 50-ms window are shown. Sliding correlations between PN spike activity and LFPs in the AL (Top) or MB (Middle) reveal no peaks at any time before, during, or after the stimulus (P = 0.31 and 0.42 for top and middle traces, respectively). There also was no significant correlation (P = 0.09) between LFPs in the MB and AL (Bottom).
PN Synchronization Is Not Phase-Locked to LFPs. If odor-specific information is encoded through a subset of coherently firing PNs that are time-locked to a particular phase of the LFP oscillations, as suggested in several reports, then temporal analysis should reveal such phase locking. We tested this idea by combining LFP and neural-ensemble recordings from multiple units (12) to examine the dynamics of population responses evoked by nonpheromonal stimuli (see Fig. 6, which is published as supporting information on the PNAS web site). After completing statistical sorting of all units in each ensemble (see Experimental Procedures and Supporting Methods), presumptive PNs were separated from LNs according to previously established physiological criteria (11). We chose to use a prolonged (5 s) stimulus pulse because this protocol reliably evoked a period of continuous oscillatory LFP activity along with distinct temporal patterning in each of the units in the ensembles. Although LFP oscillations were also observed sporadically both before and after the olfactory stimulus, LFP amplitude increased significantly during the olfactory response (Fig. 6A). In all cases (n = 17 trials in three PN ensembles), this increase in the LFP was initiated shortly after stimulus onset, but well before the onset of PN spiking activity (Fig. 6B). Fourier analysis of the LFPs showed further that spectral peaks remained in the same frequency range with or without olfactory stimulation (Fig. 6C).
If network interactions involving PNs in a given glomerulus give rise to LFP oscillations, then a phasic relationship would be expected between the PN spikes and the LFP when these potentials are recorded together. In contrast, temporal analysis showed that during response epochs, when unit and LFP activity occurred together, PN spikes did not maintain a fixed phase relationship relative to the LFP oscillations. All units that were synchronized by the olfactory stimulus were clearly not synchronized to a preferred phase of the LFP at any time before, during, or after the olfactory response (Fig. 6 D and E). This was particularly evident near response onset, when synchronous firing between PNs was most likely to occur (9, 12). The lack of correspondence between PN synchrony and LFP oscillations furthermore remained consistent over repeated trials (Fig. 6F), thus revealing no evidence for a progressive increase in oscillatory synchrony with experience (22, 23). Taken together, these findings show that although network oscillations exist in the moth AL, they do not serve as a global time reference to help coordinate the stimulus-evoked firing activity of glomerular output neurons.
Discussion
Our findings provide evidence that olfactory glomeruli use combinatorial and temporally dynamic measures to process olfactory input signals, but in the moth, the neural transformation that leads to coordinated glomerular output is not governed by underlying network rhythmicity. We found that olfactory stimulation evoked dynamic firing patterns in glomerular PNs as well as LFP oscillations in both the AL and MB, but these events were not temporally correlated. Of course, we cannot rule out the possibility that rhythmic or oscillatory behavior that is not reflected in the LFP recordings may also influence the firing properties of PNs.
Analyses of olfactory networks in a wide range of different species show that olfactory stimuli often evoke synchronous firing across populations of glomerular output neurons, but there are significant discrepancies in the reported time course of PN synchrony. Although these distinctions may arise from species differences, they may also reflect the different stimulation protocols currently used to examine odor-evoked neural representations in the brain. Using prolonged odor pulses (ranging up to several seconds), studies in locusts and honey bees showed that the timing of PN spiking was phase-locked to multiple cycles of an underlying 20- to 30-Hz oscillation (3, 8, 17, 21–23). Other studies in moths, using brief (50- to 200-ms) odor pulses, suggested that synchronous firing between PNs is instead modulated transiently by the variable time course of an intermittent stimulus (4, 9–12, 20). In the present study, we now provide direct evidence from PNs innervating functionally specified glomeruli that the patterns of odorant-evoked synchrony in the AL and LFP oscillations recorded in the same neuropil and in the MB are not temporally correlated (Fig. 6). These results are particularly significant because they are consistent with behavioral data showing that moths require a series of brief and intermittent stimulus pulses to correctly identify and ultimately locate a distant odor source (20, 24). No corresponding data are yet available for other insects, but this information is crucial to understand the behavioral context in which an animal smells and discriminates the enormous range of different scents available in its natural environment.
It is often assumed that coherent oscillations in neural networks reflect patterns of synchronized spiking across a neural population, but this idea is not universally accepted. Instead, LFPs are sometimes better characterized as the summation of subthreshold synaptic currents in the immediate vicinity of the recording electrode (13). Odor-evoked LFPs therefore do not necessarily propagate globally (5), and the results we present here support this view for several reasons. First, simultaneous LFP recordings from different sites in the AL indicate that “local” field potentials are indeed local. That is, they are not necessarily globally coherent (21), but are instead confined to the neuropil in which they are generated (Fig. 3). This result is consistent with several new findings in bees. In honey bees, LFP oscillations between the brain hemispheres are not coupled (25), and in bumblebees, odorant-evoked oscillations in the AL are localized to regions corresponding to only one or a few glomeruli (26). All of these findings are furthermore consistent with results in the vertebrate olfactory bulb (5). Second, LFP oscillations in the MB occur in the absence of an olfactory stimulus or PN firing in the AL (Fig. 5). This finding implies that LFP oscillations in the MB reflect more complex network dynamics, involving not only the AL-olfactory input to the MB but also the many intrinsic and extrinsic neurons associated with the MB itself (16). This situation is not unlike that reported in vertebrate visual pathways, where it is likely that input from the lateral geniculate nucleus alone cannot account for oscillations in visual cortex (27). Third, unlike the case in other insect species (17), LFP oscillations in the moth can be recorded in both the AL and MB (Fig. 5). Importantly, however, the LFPs in these two regions of the moth CNS were not temporally correlated (Fig. 5E). Such a correlation would be expected if LFP oscillations in the MB were driven globally by synchronized PN spiking arising in the AL. It therefore remains unclear how LFP oscillations in the MB can function as a periodic time reference to aid in the transfer of synchronized PN input from the AL.
These findings have important implications for understanding hypotheses that relate neural coherence and information coding in sensory systems. Synchronous firing is a prominent feature in the CNS of different species, and its possible functional significance in various sensory systems has been reviewed and debated extensively (1–14, 22, 23, 27–32). In the olfactory system, it has been proposed that oscillatory synchronization across subsets of PNs is functionally relevant for odor discrimination, but our data do not support this view. We found no evidence in the AL of M. sexta that odorant-evoked firing in PNs is phase-locked to network oscillations, which are prominent, but as yet poorly understood phenomena in olfactory processing networks. The fact that neurons respond to an olfactory stimulus with temporally modulated firing patterns does not necessarily mean that these patterns are part of the neural code that identifies the stimulus. Our studies instead support the long-held view that odor identity is encoded primarily in the spatial pattern of activated PNs (33–39), and that the temporal coordination of PN activity that is superimposed on the spatial pattern is reserved for tracking other important features of the stimulus (4, 11, 12, 20, 24, 40). It is also clear from numerous studies of diverse olfactory systems that the spatial arrangement of glomeruli in the primary olfactory center is largely invariant (33–39). The same cannot be said for the dynamic patterning of glomerular output activity evoked by an olfactory stimulus. Many variables, both extrinsic and intrinsic to the nervous system, can affect the timing of signals in primary olfactory circuits. For example, although often overlooked, the dynamic nature of odor is a physical property that is fundamental to the delivery of all olfactory stimuli (41). Importantly, this is true whether an animal samples actively (e.g., sniffing in vertebrates; ref. 42) or passively (flicking the olfactory appendages in arthropods; ref. 43). It is therefore critical to understand how stimulus dynamics might influence temporal patterning of olfactory responses in the brain before we can fully interpret the role of intrinsic network dynamics in olfactory information coding.
Supplementary Material
Acknowledgments
We thank W. Gronenberg and V. Pawlowski for helpful comments, and all of the members of our laboratory for many stimulating discussions. Multichannel silicon recording arrays were kindly provided by the University of Michigan Center for Neural Communication Technology (National Institutes of Health/National Institute for Biomedical Imaging and Bioengineering Grant P41 RR09754). This work was supported by National Institute on Deafness and Other Communication Disorders Grants DC-02751 (to J.G.H.) and DC-05652 (to T.A.C.).
Abbreviations: AL, antennal lobe; LFP, local field potential; MB, mushroom body; MGC, macroglomerular complex; PN, projection neuron.
References
- 1.Gelperin, A. (1999) J. Exp. Biol. 202, 1855–1864. [DOI] [PubMed] [Google Scholar]
- 2.Mori, K., Nagao, H. & Yoshihara, Y. (1999) Science 286, 711–715. [DOI] [PubMed] [Google Scholar]
- 3.Laurent, G. (1999) Science 286, 723–728. [DOI] [PubMed] [Google Scholar]
- 4.Christensen, T. A. & White, J. (2000) in The Neurobiology of Taste and Smell, eds. Finger, T. E., Silver, W. L. & Restrepo, D. (Wiley, New York), pp. 201–232.
- 5.Lam, Y.-W., Cohen, L. B., Wachowiak, M. & Zochowski, M. R. (2000) J. Neuroscience 20, 749–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorries, K. M. & Kauer, J. S. (2000) J. Neurophysiol. 83, 754–765. [DOI] [PubMed] [Google Scholar]
- 7.Sachse, S. & Galizia, C. G. (2002) J. Neurophysiol. 87, 1106–1117. [DOI] [PubMed] [Google Scholar]
- 8.Laurent, G., Stopfer, M., Friedrich, R. W., Rabinovich, M. I., Volkovskii, A. & Abarbanel, H. D. (2001) Annu. Rev. Neurosci. 24, 263–297. [DOI] [PubMed] [Google Scholar]
- 9.Lei, H., Christensen, T. A. & Hildebrand, J. G. (2002) Nat. Neurosci. 5, 557–565. [DOI] [PubMed] [Google Scholar]
- 10.Hansson, B. S. & Christensen, T. A. (1999) in Insect Olfaction, ed. Hansson, B. S. (Springer Verlag, Berlin), pp. 126–161.
- 11.Christensen, T. A. & Hildebrand, J. G. (2002) Curr. Opin. Neurobiol. 12, 393–399. [DOI] [PubMed] [Google Scholar]
- 12.Christensen, T. A., Pawlowski, V. M., Lei, H. & Hildebrand, J. G. (2000) Nat. Neurosci. 3, 927–931. [DOI] [PubMed] [Google Scholar]
- 13.Van Hooser, S. D., Hofmann, U. G., Kewley, D. T. & Bower, J. M. (2000) Neurocomputing 32–33, 591–596. [Google Scholar]
- 14.Heinbockel, T., Kloppenburg, P. & Hildebrand, J. G. (1998) J. Comp. Physiol. A 182, 703–714. [DOI] [PubMed] [Google Scholar]
- 15.Heisenberg, M. (1998) Learn. Mem. 5, 1–10. [PMC free article] [PubMed] [Google Scholar]
- 16.Strausfeld, N. J., Hansen, L., Li, Y., Gomez, R. S. & Ito, K. (1998) Learn. Mem. 5, 11–37. [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent, G. & Naraghi, M. (1994) J. Neurosci. 14, 2993–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inokuma, Y., Inoue, T., Watanabe, S. & Kirino, Y. (2001) J. Neurophysiol. 87, 3160–3164. [DOI] [PubMed] [Google Scholar]
- 19.Nikonov, A., Parker, J. M. & Caprio, J. (2002) J. Neurosci. 22, 2352–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vickers, N. J., Christensen, T. A., Baker, T. C. & Hildebrand, J. G. (2001) Nature 410, 466–470. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Orive, J., Mazor, O., Turner, G. C., Cassenaer, S., Wilson, R. I. & Laurent, G. (2002) Science 297, 359–365. [DOI] [PubMed] [Google Scholar]
- 22.Stopfer, M. & Laurent, G. (1999) Nature 402, 664–668. [DOI] [PubMed] [Google Scholar]
- 23.Stopfer, M., Bhagavan, S., Smith, B. H. & Laurent, G. (1997) Nature 390, 70–74. [DOI] [PubMed] [Google Scholar]
- 24.Murlis, J., Elkinton, J. S. & Cardé, R. T. (1992) Annu. Rev. Entomol. 37, 505–532. [Google Scholar]
- 25.Ritz, R., Galán, R. F., Szyszka, P. & Herz, A. V. M. (2001) Neurocomputing 38–40, 313–318. [Google Scholar]
- 26.Okada, K. & Kanzaki, R. (2001) Neurosci. Lett. 316, 133–136. [DOI] [PubMed] [Google Scholar]
- 27.Singer, W. & Gray, C. M. (1995) Annu Rev. Neurosci. 18, 555–586. [DOI] [PubMed] [Google Scholar]
- 28.Vaadia, E., Ahissar, E., Bergman, H. & Lavner, Y. (1991) in Neuronal Cooperativity, ed. Kruger, J. (Springer, Berlin), pp. 205–279.
- 29.Singer, W. (1999) Neuron 24, 49–65. [DOI] [PubMed] [Google Scholar]
- 30.Laurent, G. & Davidowitz, H. (1994) Science 265, 1872–1875. [DOI] [PubMed] [Google Scholar]
- 31.Murthy, V. N. & Fetz, E. E. (1996) J. Neurophysiol. 76, 3968–3982. [DOI] [PubMed] [Google Scholar]
- 32.Bazhenov, M., Stopfer, M., Rabinovich, M., Huerta, R., Abarbanel, H. D., Sejnowski, T. J. & Laurent, G. (2001) Neuron 30, 553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson, B. A., Woo, C. C., Hingco, E. E., Pham, K. L. & Leon, M. (1999) J. Comp. Neurol. 409, 529–548. [PubMed] [Google Scholar]
- 34.Galizia, C. G. & Menzel, R. (2001) J. Insect Physiol. 47, 115–130. [DOI] [PubMed] [Google Scholar]
- 35.Meister, M. & Bonhoeffer, T. (2001) J. Neurosci. 21, 1351–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belluscio, L. & Katz, L. C. (2001) J. Neurosci. 21, 2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuss, S. H. & Korsching, S. I. (2001) J. Neurosci. 21, 8396–8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spors, H. & Grinvald, A. (2002) Neuron 34, 301–315. [DOI] [PubMed] [Google Scholar]
- 39.Ng, M., Roorda, R. D., Lima, S. Q., Zemelman, B. V., Morcillo, P. & Miesenbock, G. (2002) Neuron 36, 463–474. [DOI] [PubMed] [Google Scholar]
- 40.Christensen, T. A., Waldrop, B. R. & Hildebrand, J. G. (1998) J. Neurosci. 18, 5999–6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dethier, V. G. (1987) Proc. Am. Philos. Soc. 131, 159–176. [Google Scholar]
- 42.Youngentob, S. L., Mozell, M. M., Sheehe, P. R. & Hornung, D. E. (1987) Physiol. Behav. 41, 59–69. [DOI] [PubMed] [Google Scholar]
- 43.Schmitt, B. C. & Ache, B. W. (1979) Science 205, 204–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.