Abstract
We have shown previously that mice with a targeted disruption in the stearoyl-CoA desaturase 1 gene (SCD1-/-) have increased insulin sensitivity compared with control mice. Here we show that the SCD1-/- mice have increased insulin signaling in muscle. The basal tyrosine phosphorylation of the insulin receptor and insulin receptor substrates 1 and 2 are elevated. The tyrosine phosphorylation of insulin-like growth factor-1 receptor was similar between SCD1+/+ and SCD1-/- mice. The association of insulin receptor substrates 1 and 2 with αp85 subunit of phosphatidylinositol 3-kinase as well as the phosphorylation of Akt-Ser-473 and Akt-Thr-308 are also elevated in the SCD1-/- mice. Interestingly, the mRNA levels, protein mass, and activity of the protein-tyrosine phosphatase-1B implicated in the attenuation of the insulin signal are reduced in the SCD1-/- mice, whereas the levels of the leukocyte antigen-related protein phosphatase are similar between two groups of mice. The content of glucose transporter 4 in the plasma membrane and basal as well as insulin-mediated glucose uptake are increased in the SCD1-/- mice. In addition, the muscle glycogen content and the activities of glycogen synthase and phosphorylase are increased in the SCD1-/- mice. We hypothesize that loss of SCD1 function induces increased insulin signaling at least in part by a reduction in the expression of protein-tyrosine phosphatase 1B. SCD1 could be a therapeutic target in the treatment of diabetes.
Stearoyl-CoA desaturase (SCD) is a microsomal enzyme that catalyzes the synthesis of monounsaturated fatty acids from saturated fatty acyl-CoAs. The preferred substrates for SCD are palmitoyl- and stearoyl-CoA, which are converted to palmitoleoyl- and oleoyl-CoA, respectively (1). These monounsaturated fatty acids are used as substrates for the synthesis of triglycerides, wax esters, cholesteryl esters, and membrane phospholipids (2–4). The saturated to monounsaturated fatty acid ratio affects membrane phospholipid composition, and alteration in this ratio has been implicated in a variety of disease states, including diabetes, obesity, cardiovascular disease, neurological disease, skin disorders, and cancer (5–10). For this reason, the expression of SCD is of physiological importance in normal and disease states. A single human and three mouse SCD isoforms (SCD1, SCD2, and SCD3) are well characterized (11–14). The physiological role of each SCD isoform and the reason for having three or more SCD gene isoforms in the rodent genome are currently unknown. New insights into the physiological role of the SCD1 gene and its endogenous products have come from recent studies of the asebia mouse strains (abj and ab2j) that have a naturally occurring mutation in the SCD1 gene (14–16) as well as a laboratory mouse model with a targeted disruption in the SCD1 gene (SCD1-/-) (4). Using these mouse models, we have shown that SCD1-/- mice are deficient in tissue triglycerides, cholesteryl esters, wax esters, and 1-alkyl-2,3-diacylglycerol (2–4). The SCD1-/- mice have very low levels of triglycerides in the very low-density lipoprotein and low-density lipoprotein fractions compared with the WT counterparts (2). A reduction in triglyceride synthesis and levels would lead to a decrease in generalized steatosis and improved glucose transport in insulin-sensitive tissues such as muscle and heart (17).
Impairment of glucose transport in insulin-sensitive tissues contributes to the pathogenesis of type 2 (non-insulin-dependent) diabetes mellitus (18, 19). Skeletal muscle represents the most important tissue for the maintenance of a balanced postprandial glucose homeostasis; ≈80% of insulin-stimulated glucose uptake is accounted for by muscle tissue (20). In skeletal muscle and other insulin-sensitive tissues, insulin increases glucose transport into cells by stimulating the translocation of the glucose transporter isoform 4 (GLUT4) from an intracellular pool to the plasma membrane (21, 22). The intracellular-signaling pathway by which insulin mediates glucose transport involves signal transduction through the insulin receptor (IR), whereby insulin binding to the α subunit of the IR derepresses the kinase activity in the β-subunit followed by tyrosine autophosphorylation of the β-subunit and a conformational change in the receptor structure that further increases tyrosine kinase activity toward IR substrates (IRSs) (23). IRS tyrosine phosphorylation leads to activation of phosphatidylinositol 3-kinase (PI3-kinase) and Akt/protein kinase B (PKB) (24, 25), which are key signaling transducers in insulin-mediated GLUT4 translocation, glucose uptake, and glycogen synthesis (26–28). Protein tyrosine phosphatase 1B (PTP-1B), which has been implicated in the negative regulation of insulin signaling, dephosphorylates the activated IR, thereby attenuating the insulin response (29–31). PTP-1B-/- mice have sustained insulin response because the IR remains phosphorylated and therefore activated longer than in the PTP-1B+/+ mice (32).
Previously we showed that mice with a targeted disruption in the SCD1 gene are resistant to diet-induced weight gain and have increased insulin sensitivity relative to the WT controls (33). In the current study, we have investigated the link between the loss of SCD1 function and insulin signaling in muscle. We provide evidence for significant and specific alterations in the levels of insulin-signaling components in the SCD1-/- mice as demonstrated by an increase in basal tyrosine phosphorylation of the IR, IRS-1, and IRS-2; increased association of IRS-1 and IRS-2 with PI3-kinase; increased phosphorylation of Akt/PKB; and reduction in expression and activity of PTP-1B. The link between up-regulation of the insulin signaling and basal and insulin-mediated glucose uptake as well as glycogen metabolism was also studied.
Methods
Animal Experiments. The generation of targeted SCD1-/- mice has been described (4). Prebred homozygous (SCD1-/-) and WT (SCD1+/+) male mice on an SV129 background were used. Mice were maintained on a 12-h dark/light cycle and were fed a normal nonpurified diet (5008 test diet; PMI Nutrition, Richmond, IN). Mice were housed and bred in a pathogen-free barrier facility of the Department of Biochemistry. The breeding of these animals was in accordance with the protocols approved by the animal care research committee of the University of Wisconsin-Madison. Male SCD1-/- and SCD1+/+ were killed at 12 weeks of age; gastrocnemius and soleus muscles were extracted and used throughout the study. The plasma insulin and glucose levels were determined by using kits (Linco Research Immunoassay, St. Charles, MO, and Sigma).
Evaluation of Phosphorylation Status of Insulin Signaling Cascade Proteins. The phosphorylation assays were carried out as described (34). Muscle samples were homogenized and centrifuged at 100,000 × g for 1 h in ice-cold 50 mM Hepes buffer (pH 7.4) containing 150 mM NaCl, 10 mM sodium pyrophosphate, 2 mM Na3VO4, 10 mM NaF, 2 mM EDTA, 2 mM PMSF, 5 μg/ml leupeptin, 1% Nonidet P-40, and 10% glycerol. Supernatants were collected, and protein concentration was measured with the Bradford protein assay reagent (Bio-Rad), using BSA as standard. Tissue homogenates (1 mg) were then immunoprecipitated with 4 μg of antibodies to IR, IRS-1, IRS-2, or insulin-like growth factor-1 receptor β (IGF-1Rβ) (Santa Cruz Biotechnology) for 18 h. Immunoprecipitates were washed three times by brief centrifugation and gentle suspension in ice-cold homogenization buffer plus 0.1% SDS and then were subjected to SDS/PAGE on a 10% gradient gel. Proteins were transferred and immobilized on immobile P transfer membrane. The membranes were immunoblotted with anti-phosphotyrosine antibodies (Upstate Biotechnology, Lake Placid, NY), and bands were visualized by using enhanced chemiluminescence and quantified by densitometry. To measure IRS-1- or IRS-2-associated p85 subunit of PI3-kinase, equal amounts of protein (1 mg) were immunoprecipitated with either IRS-1 or IRS-2 and then immunoblotted with antibody specific to αp85 subunit of PI3-kinase (Santa Cruz Biotechnology). Akt/PKB serine and threonine phosphorylation were measured by using antibodies to phospho-Ser-473 and phospho-Thr-308 (Cell Signaling Technology, Beverly, MA). Immunoprecipitation and Western blotting procedures are the same as described for IR, IRS-1, IRS-2, and IGF-1R tyrosine phosphorylations.
PTP-1B and Leukocyte Antigen-Related (LAR) Phosphatase Expression. Total RNA was isolated from muscle of 12-wk-old SCD1+/+ and SCD1-/- male mice by using TRIzol reagent (Invitrogen) and then analyzed by RT-PCR using PTP-1B-specific primers. Real-time quantitative PCR was performed with a Smart Cycler (Cepheid, Sunnyvale, CA) by monitoring the increase in fluorescence due to the binding of SYBR green (Roche Applied Science) to double-stranded DNA (35). The PTP-1B and LAR protein levels were assessed by immunoblotting using polyclonal antibodies against PTP-1B and LAR (Santa Cruz Biotechnology), respectively. The PTP-1B activity was measured by using p-nitrophenyl phosphate (pNPP) as substrate (36).
Determination of Plasma Membrane GLUT4 Levels, Glucose Uptake, and Glucose Oxidation. Muscle plasma membranes were prepared from muscle of SCD1-/- and SCD1+/+ mice, and GLUT4 levels were determined as described (37). In vivo glucose uptake assay was carried out as described (38). Mice were anesthetized and 0.2 μCi (1 μCi = 37 kBq) of 2-deoxy-d-[1-14C]glucose (55 mCi/ mmol) and 0.8 μCi of [1-3H]mannitol (20 Ci/mmol) per 20 g of body weight were administered into the tail vein of SCD1+/+ and SCD1-/- mice. [1-3H]Mannitol was used to measure the extracellular space. The blood and the muscles were isolated after 25 min. The samples were digested with 1 M KOH followed by neutralization with 1 M HCl. The scintillation mixture was added and radioactivity was quantified in a liquid scintillation counter. The 2-deoxyglucose uptake was calculated as the difference between the total muscle radioactivity and the radioactivity of the muscle extracellular space. In vitro glucose uptake assay was carried out as described (39). The media used for muscle incubation were equilibrated with 95% O2/5% CO2 before use, and all incubations were carried out at 37°C under an atmosphere of 95% O2/5% CO2. After incubation the muscle and aliquots of incubation medium were digested in 1 M KOH and the cellular uptake of radioactive 2-deoxyglucose was determined as described above. Glucose oxidation was determined in thin slices of gastrocnemius muscle as described (40).
Measurement of Glycogen. Glycogen content in muscle was measured as described (41). To determine glycogen accumulation, sections of gastrocnemius muscle 2–3 mm in diameter were fixed in buffered 10% formalin and, after dehydration, were embedded in Paraplast. Sections (4–6 μm thick) were cut, dewaxed, and rehydrated, and standard periodic acid/Schiff (PAS) reaction was performed. Glycogen synthase and phosphorylase activities were assayed in gastrocnemius muscle homogenates as described (42).
Statistical Analysis. All data are presented as mean ± SD (n = 6). Statistical analyses were performed by using the unpaired Student's t test.
Results
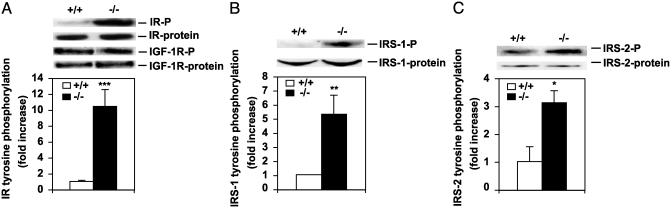
Increased Basal Tyrosine Phosphorylation of IR and IRSs in SCD1-/- Mice. We first measured the plasma glucose and insulin levels of SCD1-/- and SCD1+/+ mice. The nonfasting plasma insulin levels were lower in the SCD1-/- mice than in the SCD1+/+ (SCD1-/-, 0.645 ± 0.053 ng/ml; SCD1+/+, 1.245 ± 0.106 ng/ml; P < 0.005). The glucose levels also tended to be lower in the SCD1-/- mice compared with the controls (SCD1-/-, 88.8 ± 1.96; SCD1+/+, 111.7 ± 7.4). To assess the phosphorylation status of the IR, immunoprecipitated IR was subjected to Western blotting with anti-phosphotyrosine antibodies (Fig. 1A). Densitometric analysis revealed that despite the lower levels of plasma insulin, the basal IR tyrosine phosphorylation was 10-fold higher (P < 0.0005) in the muscle of the SCD1-/- mice compared with the WT mice. To determine whether the phosphorylation of the proximal elements of the insulin-signaling cascade was also increased in the basal state, we assessed the degree of IRS-1 and IRS-2 tyrosine phosphorylation as well as the protein levels. IRS-1 tyrosine phosphorylation was 5-fold higher (P < 0.005) in the muscle of SCD1-/- mice compared with the WT mice (Fig. 1B). IRS-2 tyrosine phosphorylation was 3-fold higher (P < 0.01) in the SCD1-/- mice than in controls (Fig. 1C). There was no significant difference in the IR and IRS-2 protein levels between the two groups of mice. The IRS-1 protein levels were 1.5-fold higher (P < 0.05) in the SCD1-/- mice. To determine whether the increased phosphorylation is specific to the insulin-signaling pathway, we examined the phosphorylation status of IGF-1R, which upon tyrosine phosphorylation is known to regulate signaling by means of the shc/ mitogen-activated protein kinase, leading to metabolic changes in muscle (43–45). As shown in Fig. 1 A, the tyrosine phosphorylation levels of the IGF-1R and the protein levels were similar in SCD1+/+ and SCD1-/- mice. Thus, increased IR, IRS-1, and IRS-2 tyrosine phosphorylation is consistent with being specific to the insulin-signaling pathway in the SCD1-/- mice.
Fig. 1.
Immunoblots and densitometric quantification of IR, IGF-1R, IRS-1, and IRS-2 phosphorylation status and protein levels in muscle of SCD1+/+ and SCD1-/- mice. Tyrosine-phosphorylated forms are indicated by -P. (A) IR-P and IGF-1R-P and protein levels. (B) IRS-1-P and protein. (C) IRS-2-P and protein. *, P < 0.01; **, P < 0.005; ***, P < 0.0005 vs. controls.
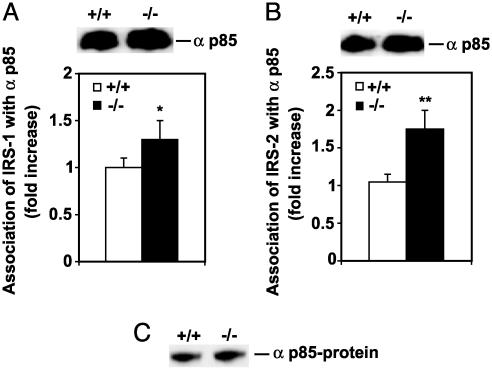
Increased αp85 Association with the IRSs in SCD1-/- Mice. It is known that when tyrosine residues of IR substrates are phosphorylated, they associate with αp85 subunit of PI3-kinase, resulting in its activation (46) and involvement in insulin signal transduction. The association of αp85 subunit of PI3-kinase with IRS-1 (Fig. 2A) and IRS-2 (Fig. 2B) was 1.3-(P < 0.05,) and 1.7-fold (P < 0.01), respectively, higher in the SCD1-/- mice compared with SCD1+/+ mice. There was no change in the levels of αp85 protein (Fig. 2C).
Fig. 2.
Association of IRS-1 and IRS-2 with the αp85 subunit of PI3-kinase and αp85 abundance in muscle. (A) Association of IRS-1 with αp85. (B) Association of IRS-2 with αp85. (C) The p85 protein level. Data are means ± SD. *, P < 0.05; **, P < 0.01 vs. controls.
Reduced PTP-1B Expression in SCD1-/- Mice. Protein-tyrosine phosphatases, particularly PTP-1B, play an important role in regulating the phosphorylation status of proteins involved in insulin signaling. To investigate the possible role of PTP-1B in signal transduction, experiments were conducted to measure the expression, protein mass, and activity of PTP-1B in muscle of SCD1-/- and SCD1+/+ mice. RT-PCR analysis using total RNA prepared from muscle shows >66% reduction (P < 0.001) in PTP-1B mRNA expression in SCD1-/- compared with SCD1+/+ mice (Fig. 3A). The protein mass was analyzed by using a specific anti-PTP-1B polyclonal antibody. Fig. 3B shows that the PTP-1B protein levels were 42% lower (P < 0.001) in SCD1-/- compared with SCD1+/+ mice. Consistent with reduction in protein mass, the PTP-1B activity in muscle of SCD1-/- was reduced by 49% (P < 0.001) compared with that in muscle of control mice (Fig. 3C). To determine whether the down-regulation of PTP-1B is specific to the insulin-signaling pathway in the SCD1-/- mice, we examined the protein levels of the LAR protein phosphatase, a protein tyrosine phosphatase that has a wide tissue distribution and is implicated in negatively regulating the IR signaling (47). As shown in Fig. 3A, the protein levels of LAR were similar in SCD1+/+ and SCD1-/- mice.
Fig. 3.
mRNA, protein, and activity of PTP-1B in muscle of SCD1+/+ and SCD1-/- mice. (A) PTP-1B mRNA levels. (B) PTP-1B and LAR protein levels along with combined densitometric analysis. (C) PTP-1B activity. *, P < 0.001 vs. controls.
Increased Phosphorylation of Akt/PKB in the SCD1-/- Mice. To investigate insulin-signaling status downstream of PI3-kinase, we examined the phosphorylation status of Ser-473 and Thr-308 of Akt/PKB, a key serine/threonine kinase, which mediates many metabolic effects of insulin, including activation of GLUT4 translocation to the plasma membrane (24–25). The immunoblot analysis in Fig. 4A and the densitometric analysis show that Ser-473 (Fig. 4B) and Thr-308 (Fig. 4C) phosphorylation was 6-fold (P < 0.005) and 5-fold higher (P < 0.005), respectively, in SCD1-/- mice compared with SCD1+/+ mice, indicating that phosphorylation of Akt/PKB was significantly increased in the SCD1-/- mice. Immunoblotting for Akt mass (Fig. 4A) did not show significant differences between the SCD1-/- and SCD1+/+ mice.
Fig. 4.
Akt/PKB serine and threonine phosphorylation in muscle of SCD1+/+ and SCD1-/- mice. (A) Representative immunoblot. (B and C) Densitometric quantification. *, P < 0.005 vs. controls.
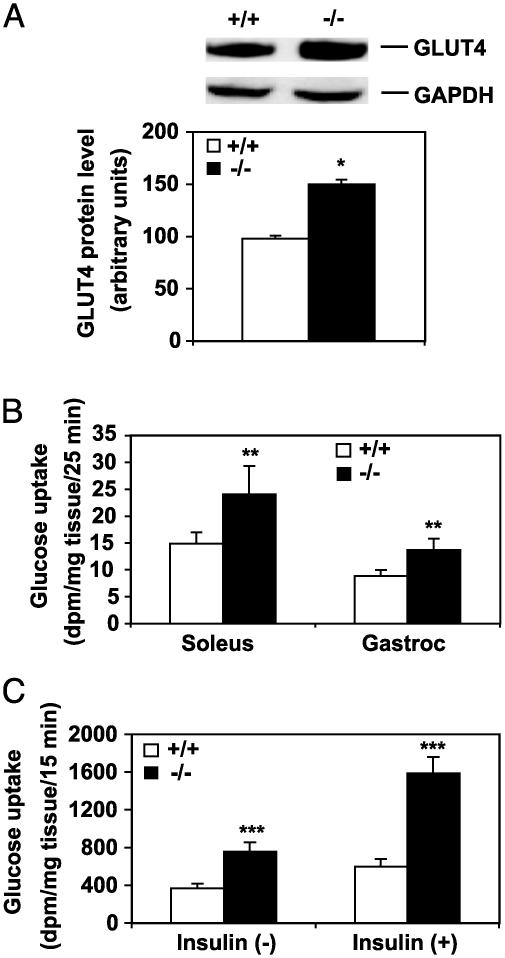
Increased Levels of GLUT4 in Plasma Membrane of SCD1-/- Mice. The elevation of the insulin-signaling components would be expected to lead to increased uptake of glucose into cells by the glucose transporter GLUT4. We determined by Western blotting the changes in the levels of GLUT4 in the plasma membranes isolated from muscle of SCD1-/- and SCD1+/+ mice (Fig. 5A). Densitometric analysis shows that the GLUT4 levels in the plasma membrane of SCD1-/- mice are 1.5-fold higher (P < 0.05) compared with SCD1+/+ mice. The GAPDH antibody was used as control for loading, and as shown the GAPDH levels were not altered in the plasma membranes of the SCD1-/- and SCD1+/+ mice. We then measured in vivo deoxyglucose uptake in muscle to determine whether the increase in GLUT4 levels in the plasma membrane of the SCD1-/- mice results in increased glucose uptake. Radioactive deoxyglucose was injected i.v. and its distribution in muscle of the SCD1-/- and SCD1+/+ mice was determined. Radioactive mannitol was used as an internal control. There was a 1.5-fold (P < 0.01) and 1.7-fold (P < 0.01) increase in 2-deoxyglucose content in the gastrocnemius and soleus muscles, respectively, of SCD1-/- mice compared with the SCD1+/+ mice (Fig. 5B). To determine whether muscle from SCD1-/- mice demonstrated increased insulin responsiveness, we performed insulin-stimulated glucose uptake experiments in isolated soleus muscle of both SCD1-/- and SCD1+/+ mice. As shown in Fig. 5C, insulin-mediated glucose uptake was 2.1-fold higher (P < 0.001) in the soleus muscle from SCD1-/- mice compared with 1.6-fold (P < 0.001) in the SCD1+/+ mice (Fig. 5C). Thus, soleus muscle from SCD1-/- mice demonstrated a pronounced elevation of the effects of insulin on glucose uptake.
Fig. 5.
Expression and quantification of GLUT4 and glucose uptake in muscle of SCD1-/- and SCD1+/+ mice. (A) Representative immunoblot of GLUT4 protein expression along with combined densitometric analysis. (B) Glucose uptake measured in vivo in soleus and gastrocnemius (Gastroc) muscles. (C) Basal and insulin-stimulated glucose uptake in isolated soleus muscle from control and SCD1-/- mice. *, P < 0.05; **, P < 0.01; ***, P < 0.0001 vs. controls.
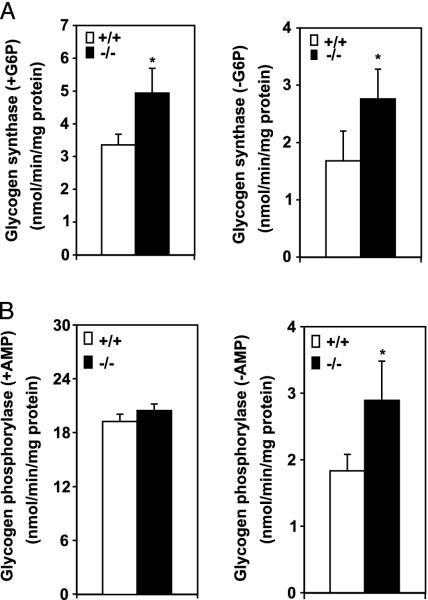
Increased Glycogen Synthesis and Turnover in SCD1-/- Mice. To determine whether increased glucose uptake leads to increased glycogen synthesis, we measured the activities of two key enzymes in glycogen metabolism: glycogen synthase and glycogen phosphorylase. Both the total and active forms of glycogen synthase were 1.5-fold (P < 0.05) and 1.6-fold higher (P < 0.05), respectively, in the muscle of SCD1-/- mice (Fig. 6A). Total glycogen phosphorylase activity was similar in the SCD1-/- and WT mice, but the activity of the active form of glycogen phosphorylase as measured in the absence of AMP was 1.5-fold higher (P < 0.05) in SCD1-/- mice (Fig. 6B). The glucose oxidation was similar in the two groups of mice (SCD1+/+, 0.85 ± 0.9 vs. SCD1-/-, 0.89 ± 0.11 mmol/h per g of tissue) despite increased glycogen synthesis and turnover in the SCD1-/- mice.
Fig. 6.
Enzyme activities in muscle of SCD1-/- and SCD1+/+ mice. (A) Glycogen synthase activities in muscle, in the presence and absence of glucose 6-phosphate. (B) Glycogen phosphorylase activities in the presence and absence of AMP. *, P < 0.05 vs. controls.
To determine whether increased glycogen synthesis resulted in net glycogen accumulation, we measured glycogen content in the muscle of SCD1-/- and SCD1+/+ mice. Chemical determination of glycogen showed 1.8-fold higher (P < 0.001) glycogen content in muscle of SCD1-/- mice (Fig. 7A). The increased glycogen content was confirmed by light microscopy examination, which showed that the muscle of SCD1-/- has more red granules with PAS staining (Fig. 7C) than the muscle of SCD1+/+ mice (Fig. 7B).
Fig. 7.
Muscle glycogen content and light microscopy of muscle tissue stained with PAS. (A) Chemical determination of glycogen in muscle. *, P < 0.001 vs. controls. (B and C) Muscle from control mice (B) and SCD1-/- mice (C) stained with PAS. The arrows point to the red glycogen granules. (×600.)
Discussion
These studies establish a critical role of SCD1 in the insulin signal transduction in muscle. The deletion of SCD1 resulted in up-regulation of the insulin-signaling components that can account for increased basal and insulin-mediated glucose uptake.
We previously observed lower levels of fasting plasma insulin and glucose in the SCD1-/- compared with the SCD1+/+ mice (33). However, glucose tolerance tests suggested that the SCD1-/- mice were more insulin responsive than the SCD1+/+ mice (33). We show in this study that loss of SCD1 function in mice leads to increased basal state tyrosine phosphorylation of IR, IRS-1, and IRS-2 in muscle despite lower levels of plasma insulin. The increase in phosphorylation is not global because we found that the tyrosine phosphorylation of IGF-1R signaling via the shc/mitogen-activated protein kinase pathway but not the IRS-1 pathway (43–45) was not increased in the SCD1-/- mice. The increased phosphorylation of the insulin-signaling components is therefore specific to the insulin-signaling pathway in the SCD1-/- mice.
There are several mechanisms by which SCD1 deficiency could lead to increased basal tyrosine autophosphorylation of the IR, despite lower levels of plasma insulin in the SCD1-/- mice. The mechanism that is consistent with our results is that loss of SCD1 function results in the down-regulation of the expression of PTP-1B, an enzyme that would catalyze the rapid dephosphorylation of the IR and IRS-1, attenuating the insulin response. A number of PTPs have been identified that catalyze the dephosphorylation of the IR in insulin-sensitive tissues and are up-regulated in states of insulin resistance (29–31). Most attention has focused on the cytoplasmic tyrosine phosphatase (PTP-1B). We found reduced expression of the PTP-1B mRNA, protein levels, and enzyme activity in the SCD1-/- mice. Consistent with our observations, PTP-1B-/- mice exhibit increased basal tyrosine phosphorylation of the IR and IRS-1 in muscle (32). The protein levels of LAR, a protein tyrosine phosphatase that is implicated in negatively regulating the IR signaling, were similar in SCD1+/+ and SCD1-/- mice, and indeed LAR-/- mice display no improvement in insulin sensitivity (47). The results presented show that the insulin-signaling pathway involving the PTP-1B is specifically targeted and it is reasonable to propose at this time that down-regulation of the PTP-1B expression and activity is responsible for the sustained IR autophosphorylation despite reduced levels of plasma insulin in the SCD1-/- mice. We found that insulin-mediated glucose uptake was also higher in the soleus muscle from SCD1-/- mice, suggesting that the IR is more responsive to insulin in the SCD1-/- mice than in SCD1+/+ mice (Fig. 5C). The PTP-1B-/- mice also show increased insulin sensitivity and, like the SCD1-/- mice, are resistant to diet-induced obesity. Thus, the phenotypes exhibited by the PTP-1B-/- mice in many ways are similar to those of the SCD1-/- mice. Further experiments will be required to determine how SCD1 deficiency leads to down-regulation of the PTP-1B expression.
The series of protein phosphorylations on the signaling molecules downstream of the IR culminates in the uptake of glucose into cells by the glucose transporter GLUT4. The mechanism by which GLUT4-containing vesicles become activated and dock at the plasma membrane remains a controversial issue. However, Akt activated by PI3-kinase phosphorylation has been implicated in the process (26). Akt also appears to participate in the insulin-signaling pathway by phosphorylating glycogen synthase kinase 3 to promote glycogen synthesis by glycogen synthase (48, 49). We found increased association of PI3-kinase with IRS-1 and IRS-2, increased serine and threonine phosphorylation of total Akt, as well as a significant increase in both total and active form of glycogen synthase in the muscle of SCD1-/- mice. The glycogen content was higher in the muscle of SCD1-/- mice (Fig. 7A), and histological analysis of muscle tissue revealed the presence of more glycogen granules in SCD1-/- mice than in WT (Fig. 7C). These results indicate that increased insulin signaling followed by increased glucose transport and increased glycogen synthesis ultimately lead to increased glycogen accumulation in the muscle of SCD1-/- mice. Interestingly, we also found that muscle glycogen phosphorylase activity was also higher in the SCD1-/- mice (Fig. 6B). Increased glycogen phosphorylase activity would have been expected to promote glycogenolysis. However, overexpression of glycogen phosphorylase in cultured human skeletal muscle cells increased the level of glycogen synthase (50), suggesting that the amount of glycogen synthase and phosphorylase are controlled in a reciprocal manner by glycogen levels. Thus, the increase in both glycogen synthase and phosphorylase activities would also imply that there is increased glycogen metabolism in the SCD1-/- mice. This observation together with our previous results showing that SCD1-/- mice have a higher metabolic rate and oxygen consumption both in the dark and in the light (33) provide strong evidence that SCD1 expression plays a major role in lipid and carbohydrate metabolism.
In summary, the present work demonstrates that SCD1 deficiency leads to increased insulin signaling and glycogen metabolism. The increased tyrosine phosphorylation of IR and IRS-1 and the activation of Akt/PKB are known events that lead to increased glucose uptake and glycogen accumulation in muscle. The mechanism of sustained autophosphorylation of the IR seems to be due to the down-regulation of the PTP-1B activity in the SCD1-/- mice. Reduction in PTP-1B activity has been associated with increased insulin signaling and reduction in insulin resistance. Thus, the SCD1 gene may be a potential target in the treatment of insulin resistance and diabetes.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO162388 (to J.M.N.) and in part by funds from Xenon Genetics, Inc. (to J.M.N.).
Abbreviations: SCD, stearoyl-CoA desaturase; PTP-1B, protein-tyrosine phosphatase 1B; IR, insulin receptor; IRS, IR substrate; IGF-1R, insulin-like growth factor-1 receptor; PI3-kinase, phosphatidylinositol 3-kinase; LAR, leukocyte antigen-related; GLUT4, glucose transporter isoform 4; PAS, periodic acid/Schiff; PKB, protein kinase B.
References
- 1.Enoch, H. G. & Strittmatter, P. (1978) Biochemistry 17, 4927-4932. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki, M., Kim, Y.-C., Gray-Keller, M. P., Attie, A. D. & Ntambi, J. M. (2000) J. Biol. Chem. 275, 30132-30138. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki, M., Kim, Y.-C. & Ntambi, J. M. (2001) J. Lipid Res. 42, 1018-1024. [PubMed] [Google Scholar]
- 4.Miyazaki, M., Man, W. C. & Ntambi, J. M. (2001) J. Nutr. 131, 2260-2268. [DOI] [PubMed] [Google Scholar]
- 5.Storlien, L. H., Jenkins, A. B., Chisholm, D. J., Pascoe, W. S., Khouri, S. & Kraegen, E. W. (1991) Diabetes 40, 280-289. [DOI] [PubMed] [Google Scholar]
- 6.Jones, B. H., Maher, M. A., Banz, W. J., Zemel, M. B., Whelan, J., Smith, P. J. & Moustaid, N. (1996) Am. J. Physiol. 271, E44-E49. [DOI] [PubMed] [Google Scholar]
- 7.Pan, D. A., Hulbert, A. J. & Storlein, L. H. (1994) J. Nutr. 124, 1555-1565. [DOI] [PubMed] [Google Scholar]
- 8.Pettegrew, J. W., Panchalingam, K., Hamilton, R. L. & McClur, R. J. (2001) Neurochem. Res. 26, 771-782. [DOI] [PubMed] [Google Scholar]
- 9.Solans, R., Motta, C., Sola, R., La Ville, A. E., Lima, J., Simeon, P., Montella, N., Armadans-Gil, L., Fonollosa, V. & Vilardell, M. (2000) Arthritis Rheum. 43, 894-900. [DOI] [PubMed] [Google Scholar]
- 10.Agatha, G., Hafer, R. & Zintl, F. (2001) Cancer Lett. 173, 139-144. [DOI] [PubMed] [Google Scholar]
- 11.Ntambi, J. M., Buhrow, S. A., Kaestner, K. H., Christy, R. J., Sibley, E., Kelly, T. J., Jr., & Lane, M. D. (1988) J. Biol. Chem. 263, 17291-17300. [PubMed] [Google Scholar]
- 12.Kaestner, K. H., Ntambi, J. M., Kelly, T. J., Jr., & Lane, M. D. (1989) J. Biol. Chem. 264, 14755-14761. [PubMed] [Google Scholar]
- 13.Bene, H., Lasky, D. & Ntambi, J. M. (2001) Biochem. Biophys. Res. Commun. 284, 1194-1198. [DOI] [PubMed] [Google Scholar]
- 14.Zhang, L., Ge, L., Parimoo, S., Stenn, K. S. & Prouty, S. M. (1999) Biochem. J. 340, 255-264. [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng, Y., Eilertsen, K. J., Ge, L., Zhang, L., Sundberg, J. P., Prouty, S. M., Stenn, K. S. & Parimoo, S. (1999) Nat. Genet. 23, 268-270. [DOI] [PubMed] [Google Scholar]
- 16.Zheng, Y., Prouty, S. M., Harmon, A., Sundberg, J. P., Stenn, K. S. & Parimoo, S. (2001) Genomics 71, 182-191. [DOI] [PubMed] [Google Scholar]
- 17.Unger, R. H. (2002) Annu. Rev. Med. 53, 319-336. [DOI] [PubMed] [Google Scholar]
- 18.Cline, G. W., Petersen, K. F., Krssak, M., Shen, J., Hundal, R. S., Trajanoski, Z., Inzucchi, S., Dresner, A., Rothman, D. L. & Shulman, G. I. (1999) N. Engl. J. Med. 341, 240-246. [DOI] [PubMed] [Google Scholar]
- 19.Garvey, W. T., Huecksteadt, T. P., Matthaei, S. & Olefsky, J. M. (1988) J. Clin. Invest. 81, 1528-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baron, A. D., Brechtel, G., Wallace, P. & Edelman, S. V. (1988) Am. J. Physiol. 255, E769-E774. [DOI] [PubMed] [Google Scholar]
- 21.Hirshman, M. F., Goodyear, L. J., Wardzala, L. J., Horton, E. D.& Horton, E. S. (1990) J. Biol. Chem. 265, 987-991. [PubMed] [Google Scholar]
- 22.Cushman, S. W. & Wardzala, L. J. (1980) J. Biol. Chem. 255, 4758-4762. [PubMed] [Google Scholar]
- 23.Withers, D. J. & White, M. (2000) Endocrinology 141, 1917-1921. [DOI] [PubMed] [Google Scholar]
- 24.Holman, G. D. & Kasuga, M. (1997) Diabetologia 40, 991-1003. [DOI] [PubMed] [Google Scholar]
- 25.Kohn, A. D., Kovacina, A. S. & Roth, R. A. (1995) EMBO J. 14, 4288-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohn, A. D., Summers, S. A., Birnbaum, M. J. & Roth, R. A. (1996) J. Biol. Chem. 271, 31372-31378. [DOI] [PubMed] [Google Scholar]
- 27.Tanti, J. F., Grillo, S., Gremeaux, T., Coffer, P. J., Van Obberghen, E. & Le Marchand-Brustel, Y. (1997) Endocrinology 138, 200-210. [DOI] [PubMed] [Google Scholar]
- 28.Thompson, A. L., Lim-Fraser, M. Y. C., Kraegen, E. W. & Cooney, G. J. (200) Am. J. Physiol. 279, E577-E584. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad, F., Azevedo, J. L., Cortright, R., Dohm, G. L. & Goldstein, B. J. (1997) J. Clin. Invest. 100, 449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad, F. & Goldstein, B. J. (1995) Metabolism 44, 1175-1184. [DOI] [PubMed] [Google Scholar]
- 31.Kenner, K. A., Hill, D. E., Olefsky, J. M. & Kusuri, J. (1993) J. Biol. Chem. 268, 25455-25462. [PubMed] [Google Scholar]
- 32.Elchebly, M., Payette, P., Michaliszyn, E., Cromlish, W., Collins, S., Loy, A. L., Normandin, D., Cheng, A., Himms-Hagen, J., Chan, C.-C., et. al. (1999) Science 283, 1544-1548. [DOI] [PubMed] [Google Scholar]
- 33.Ntambi, J. M., Miyazaki, M., Stoehr, J. P., Lan, H., Kendziorski, C. M., Yandell, B. S., Song, Y., Cohen, P., Friedman, J. M. & Attie, A. D. (2002) Proc. Natl. Acad. Sci. USA 99, 11482-11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dominici, F. P., Diaz, G. A., Bartke, A., Kopchick, J. J. & Turyn, D. (2000) J. Endocrinol. 166, 579-590. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki, M., Gomez, F. E. & Ntambi, J. M. (2002) J. Lipid Res. 43, 2146-2154. [DOI] [PubMed] [Google Scholar]
- 36.Shimuzu, S., Maegawa, H., Egawa, K., Shi, K., Bryer-Ash, M. & Kasiwagi, A. (2002) Endocrinology 143, 4563-4569. [DOI] [PubMed] [Google Scholar]
- 37.Agote, M., Goya, L., Ramos, S., Alvarez, C., Gavete, M. L., Pascual-Leone, A. M. & Escriva, F. (2001) Am. J. Physiol. 281, E1101-E1109. [DOI] [PubMed] [Google Scholar]
- 38.Dobrzyn, A. & Gorski, J. (2002) Am. J. Physiol. 281, E277-E285. [DOI] [PubMed] [Google Scholar]
- 39.Turinsky, J., Nagel, W., Elmendorf, J. S., Damrau-Abney, A. & Smith, T. R. (1996) Biochem. J. 313, 199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaushik, V. K., Young, M. E., Dean, D. J., Kurowski, T. G., Saha, A. K. & Ruderman, N. B. (2001) Am. J. Physiol. 281, E335-E340. [DOI] [PubMed] [Google Scholar]
- 41.Lo, S., Russell, J. C. & Taylor, A. W. (1970) J. Appl. Physiol. 28, 234-236. [DOI] [PubMed] [Google Scholar]
- 42.Golden, S., Wals, P. A. & Katz, J. (1977) Anal. Biochem. 77, 436-445. [DOI] [PubMed] [Google Scholar]
- 43.Chow, J. C., Condorelli, G. & Smith, R. J. (1998) J. Biol. Chem. 273, 4672-4680. [DOI] [PubMed] [Google Scholar]
- 44.Liu, J. P., Baker, J., Perkins, A. S., Robertson, E. J. & Efstratiadis, A. (1993) Cell 75, 59-72. [PubMed] [Google Scholar]
- 45.Di Cola, G., Cool, M. H. & Accili, D. (1997) J. Clin. Invest. 99, 2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Withers, D. J., Gutierrez, J. S., Towery, H., Burks, D. J., Ren, J. M., Previs, S., Zhang, Y., Bernal, D., Pons, S., Shulman, G. I., et al. (1998) Nature 391, 900-904. [DOI] [PubMed] [Google Scholar]
- 47.Mooney, R. A. & LeVea, C. M. (2003) Curr. Top. Med. Chem. 3, 809-817. [DOI] [PubMed] [Google Scholar]
- 48.Cross, D. A. E., Alessi, D. R., Cohen, P., Andjelkovich, M. & Hemmings, B. A. (1995) Nature 378, 785-789. [DOI] [PubMed] [Google Scholar]
- 49.Lochhead, P. A., Coghlan, M., Rice, S. Q. J. & Sutherland, C. (2001) Diabetes 50, 937-946. [DOI] [PubMed] [Google Scholar]
- 50.Baque, S., Guinovart, J. J. & Gomez-Foix, A. M. (1996) J. Biol. Chem. 271, 2594-2598. [DOI] [PubMed] [Google Scholar]