Abstract
Increasing evidence suggests that climate change affects the timing of breeding in birds, but there is less evidence to show how such changes affect the population dynamics of birds overall. Over the past 43 years, song sparrows (Melospiza melodia) on Mandarte Island, British Columbia, Canada have not shown an advance in breeding date in response to global warming. However, this population did show considerable annual variation in timing of breeding correlated with the El Niño Southern Oscillation. Birds bred earlier in warmer El Niño years and later in colder La Niña years. Early breeding strongly increased reproductive output. However, annual variation in timing of breeding had little effect on population growth, perhaps because the population is strongly regulated by the rate of recruitment by juveniles. The juvenile recruitment rate declined with increasing population density but showed little response to climate. These findings suggest that populations will vary in response to climate change depending on how climate affects the demographic parameters that contribute most to population growth.
Variation in climatic conditions can have wide-ranging effects on local weather patterns (1, 2) and ecological processes (3). For example, a positive shift in the North Atlantic Oscillation during the last two decades has coincided with warmer, wetter winters over Northern Europe (4), advances in plant phenology (5, 6), and an earlier spring emergence of some species of insects (7). Studies also show that the North Atlantic Oscillation can influence the timing of breeding of European birds (8, 9). Evidence for an effect of climate change on timing of breeding in North American birds is also growing. In response to warmer spring temperatures over the past few decades, Mexican jays (Aphelocoma ultramarina) and tree swallows (Tachycineta bicolor) have advanced their breeding dates by ≈9–10 days (refs. 10 and 11; see also refs. 12 and 13).
The tendency to breed earlier in temperate-zone birds is expected under conditions of increased food supply and more favorable spring climate (14–16). Thus, further advances in the timing of breeding are possible given that temperatures are expected to continue rising as carbon dioxide levels increase (17). To the degree that breeding date affects reproductive output, we might also expect that climatic warming and early breeding will influence the overall dynamics of populations. Thus far, research suggests that climate change may influence population dynamics through effects on clutch size (18) and the rate of recruitment by young birds to populations (19, 20). However, it is less clear that advances in the timing of breeding in response to climate also affect population growth.
On the northern Pacific Coast of North America, the El Niño Southern Oscillation (ENSO) influences temperatures in winter and spring (2) and might therefore be expected to influence the timing of breeding in birds that rely on insects as food (14, 21). ENSO is known to affect reproduction in birds primarily through its effect on local precipitation (20, 22–24), but it has yet to be shown that ENSO affects the timing of breeding in North American birds via its effect on spring temperature. Here, we first show that, over the past 43 years, the timing of breeding by song sparrows (Melospiza melodia) on Mandarte Island, British Columbia, Canada has not advanced in response to global warming. However, timing of breeding was strongly correlated with variation in the ENSO and its effect on spring temperature. Second, we show that variation in the timing of breeding influenced annual reproductive output in the population but had little effect on population growth. This apparently was due primarily to density-dependent variation in juvenile recruitment, which acted to regulate population size (21).
Materials and Methods
Study Area and Methods. We used 32 years of data (1960–1963 and 1975–2002) from a long-term study of a population of song sparrows on Mandarte Island to assess the effects of ENSO on the median breeding date of female birds. Data from the first 4 years of the study were collected by F. Tompa (25). Mandarte Island is ≈6 hectares in size, with ≈2 hectares of shrub habitat used regularly for nesting by individually marked song sparrows. The size of the study population was estimated as the number of breeding females alive in the last 2 weeks of April each year. Because the island is small, the habitat is well dissected by trails, and all birds are individually identifiable, we assumed that our spring counts were without error. Throughout the March–July breeding period, we visited territories approximately every 5 days to record the behavior of individual females, locate their nests, and count the number of eggs laid and young fledged. We estimated the date the first egg was laid by each female annually by observing nests during the laying stage or back dating from the day of hatching. We then used the median date of first egg for all first nests as our estimate of the annual timing of breeding for the population. Annual reproductive success was estimated as the total number of young fledged per female breeder. We excluded from analyses all females and nests with access to supplemental food in 1979 and 1985. We estimated the rate of juvenile recruitment as the proportion of juvenile females fledged in year t that survived to the last 2 weeks of April in year t + 1, assuming a 50:50 sex ratio in fledglings. We focused on females in our analysis because they are the limiting sex in this population (21). Recruitment rates are absent from the early years of the study, and therefore n (number of years with observations) is slightly lower for recruitment analyses. Population growth (λ) was determined as female population size in year t + 1/female population size in year t. Further details of the methods used to band individuals, find and follow nesting attempts, and estimate demographic rates are given by Arcese et al. (21).
Statistical relationships among climate, reproductive parameters, and population size were estimated by using general linear models. All variables were approximately normally distributed except for the annual rate of juvenile recruitment, which was right-skewed. We therefore normalized juvenile recruitment by square-root transformation but used raw values in all other tests. The residuals of all models were approximately normally distributed. We tested for potential biases in our results as a consequence of including data from 1960 to 1963, because the main data set was collected after 1974. Our results were not markedly affected by including early data. We therefore report results based on the entire study period. We report simple regression equations for many analyses to facilitate their subsequent use by others.
Climate Data. Weather data were obtained from the daily summaries collected by the Olga weather station (National Oceanic and Atmospheric Administration, U.S. Department of Commerce) located on Orcas Island, 32 km east of Mandarte Island. For our analysis of reproduction we examined cumulative precipitation (cm) and the cumulative degree days of warming (°F) (DDW) recorded from January to April each year. DDW was calculated as 65°F - [(daily maximum temperature °F + daily minimum temperature °F)/2], summed over all days from January to April (National Climate Prediction Center, www.noaa.gov). Thus, higher values of DDW indicate colder spring temperatures. Although the period of January–April is not representative of “spring” in much of North America, the extreme south coast of British Columbia experiences a mild climate with a growing season that begins in late January, marked by the emergence of annual and perennial plants near sea level. Because song sparrows on Mandarte Island commence breeding as early as late February (21), we considered it appropriate to summarize spring temperature and precipitation for January–April. We summed annual DDW for the period of November–March to estimate the effect of winter–spring temperatures on juvenile recruitment because this is the period over which substantial juvenile dispersal and mortality occurs (26).
The Southern Oscillation index (SOI) is typically calculated based on monthly shifts in the air pressure difference between Tahiti and Darwin (Australian Commonwealth Bureau of Meteorology www.bom.gov.au/climate/current/soihtml.shtml is the source used here). Large negative values of SOI indicate El Niño conditions, whereas large positive values indicate La Niña conditions. We used the mean monthly SOI value for the period of January–April each year for analyses with timing of breeding and the following November–March for analyses with juvenile recruitment.
Results
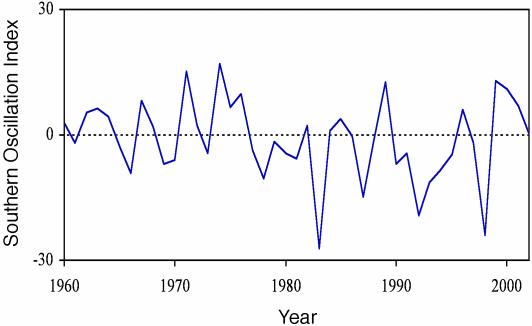
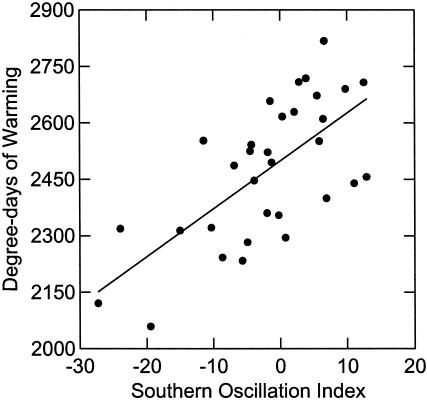
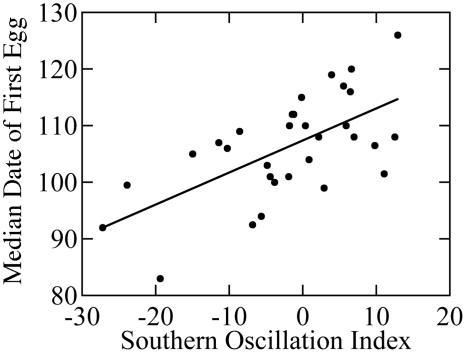
The ENSO has shown an erratic pattern over the past 43 years with major El Niño events in 1983, 1992, and 1998 and major La Niña events in 1971, 1974, 1989, and 1999 (Fig. 1). In southwest British Columbia, spring temperature was closely correlated with the ENSO (r2 = 0.46, P < 0.001, n = 31; Fig. 2). Temperatures were warmer in El Niño years and colder in La Niña years. In contrast, spring rainfall was unrelated to the SOI (r2 = 0.04, P = 0.39, n = 31). The annual median lay date varied by 43 days over the study period, occurring as early as March 23 (Julian day 83) in 1992 and as late as May 6 (Julian day 126) in 1999. Median lay date was related closely to spring temperature (r2 = 0.36, P < 0.001, n = 31; regression: lay date = 35.23 + 0.03 DDW) and to spring SOI (r2 = 0.40, P < 0.001, n = 31; Fig. 3), with the earliest breeding occurring in warm El Niño years and the latest breeding in cool La Niña years. The rate of juvenile recruitment showed little relation to temperature or the SOI either in the spring preceding birth (for temperature, r2 = 0.04 and P = 0.34; for SOI, r2 = 0.09 and P = 0.12; n = 27) or the following winter (for temperature, r2 = 0.02 and P = 0.51; for SOI, r2 = 0.10 and P = 0.11; n = 27). However, the juvenile recruitment rate was negatively related to adult female density (r2 = 0.40, P < 0.001, n = 27; recruitment = 0.70 - 0.005 females).
Fig. 1.
Mean annual January–April values of the SOI during the study period. Low negative values indicate El Niño conditions, and high positive values indicate La Niña conditions.
Fig. 2.
Influence of the ENSO on spring temperature in southwest British Columbia (r2 = 0.46, P < 0.001, n = 31; regression: DDW = 2,498.85 + 12.73 SOI). Higher values of DDW indicate colder spring temperatures.
Fig. 3.
Influence of the ENSO on annual median date of first egg (Julian days) laid in song sparrows (r2 = 0.40, P < 0.001, n = 31, lay date = 107.39 + 0.57 SOI).
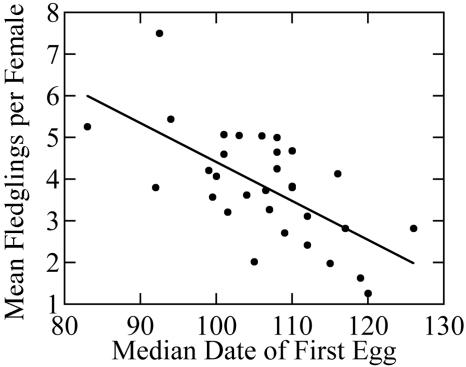
Breeding earlier had a strong positive effect on the annual number of young fledged by female song sparrows (r2 = 0.42, P < 0.001, n = 31; Fig. 4), primarily because females raised more successful nests when breeding began earlier (r2 = 0.42, P < 0.001, n = 31; successful attempts = 4.70 - 0.03 lay date). The regression equation predicts that the average female breeding in the year with the earliest median lay date will raise ≈1.29 more broods per year than females breeding in the latest year. The minimum time required for song sparrows to build a nest, lay eggs, incubate, and raise young to independence is ≈42 days (27). This interval corresponds closely to the 43-day difference between the earliest and latest median lay dates recorded during our study.
Fig. 4.
Effect of median date of first egg in year t on annual mean number of fledglings produced per female in year t (r2 = 0.42, P < 0.001, n = 31, mean fledglings = 13.75 - 0.09 lay date).
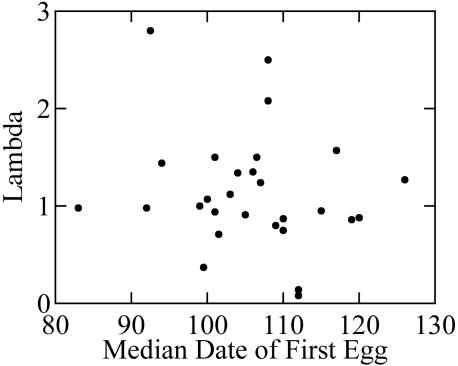
Despite the effect of timing of breeding on reproductive output, we did not find a relationship between median date of first egg in year t and population growth between year t and year t + 1 (r2 = 0.02, P = 0.48, n = 29; Fig. 5). In contrast, variation in population growth was explained largely by the rate of juvenile recruitment (r2 = 0.90, P < 0.001, n = 27, λ = -0.73 + 3.93 recruitment), with fewer juveniles being recruited on a percapita basis at higher population sizes.
Fig. 5.
Effect of median date of first egg in year t on change in population size (λ) between years t and t + 1(r2 = 0.02, P = 0.61, n = 29, λ = 1.95 - 0.007 lay date).
We detected a significant increase in spring temperature over the entire 43-year period of 1960–2002 (r2 = 0.19, P = 0.003, n = 43, DDW = 15,962.44 - 6.78 year). In contrast, we found no evidence of a directional change in lay date over the course of the study (r2 < 0.001, n = 31, P = 0.61; lay date = 170.83 - 0.03 year), despite finding close correlations between spring temperature and median lay date. However, because we lacked data for the 11-year period of 1964–1974, the analyses discussed above are not directly comparable. Therefore, we cannot rule out that an advance would have been detected if data for the entire 43-year period had been available.
Discussion
The ENSO has long been known to influence ecological processes in marine systems (28, 29), but its effects on terrestrial systems have been described only recently. One such effect concerns the influence of ENSO on reproductive success in land birds via its effect on rainfall (20, 22–24). On Mandarte Island, ENSO had little effect on rainfall but a substantial effect on temperature. The period of January–April was warmer in El Niño years and cooler in La Niña years, leading to a strong overall relationship between the SOI and timing of breeding by song sparrows. We suggest that song sparrows responded to the ENSO via one or both of two mechanisms. First, prior work on the Mandarte population has shown that the timing of breeding can be advanced with the provision of supplemental food (14, 30). Thus, earlier breeding in warmer El Niño years may be related to advances in plant and insect phenology and consequent increases in food for breeding females. Second, warmer temperatures may also influence the energy budgets of females directly, allowing them to allocate less energy to thermoregulation and more to reproduction. The experimental insulation of nest boxes also advanced breeding in great tits (Parus major), probably by facilitating the allocation of energy to reproduction (31, 32). Breeding earlier allowed female song sparrows to complete more successful nesting attempts and achieve higher reproductive output overall.
Despite observing an increase in reproductive output in association with early breeding, we were unable to detect effects of timing of breeding on population growth. The lack of a correlation between breeding date and population growth may have resulted from a number of factors but especially because density-dependent variation in the rate of juvenile recruitment acts strongly to regulate population growth (21). Recruitment declined with increasing density but showed little relation to climate. Thus, earlier breeding and enhanced reproductive output in El Niño years may act to accelerate population growth at low densities, but as population size increases, density-dependent declines in recruitment may override the effects of breeding early. Recruitment may also be influenced by predation. If predation varies among years and operates independent of climate, then this could further reduce any association between timing of breeding and population growth. Finally, it is also possible that high reproductive output in association with earlier breeding simply translated into higher rates of emigration by juveniles from Mandarte Island. Annual reproductive output on Mandarte generally exceeds that on other small islands nearby. If emigration is higher in years with early breeding at high population size, it becomes plausible that earlier breeding on Mandarte Island acts indirectly to stabilize the regional metapopulation (33).
We did not detect a long-term trend toward earlier breeding by song sparrows over the period of 1960–2002 despite evidence that January–April temperatures have increased over the same period. This result contrasts with those from two other studies of North American birds that report 9- to 10-day advances in breeding dates over a similar period (10, 11). Our inability to detect an advance in the timing of breeding by song sparrows may be due to the erratic behavior of ENSO. Although spring temperature has increased, substantial annual variation in temperature and timing of breeding exists, and longer-term data therefore may be required to demonstrate a statistically reliable trend toward earlier breeding in this population.
In conclusion, we found that annual shifts in climate influenced timing of breeding, which in turn enhanced reproductive output of individual female song sparrows in our study population. However, because climate had little influence on juvenile recruitment, the key factor regulating population size on Mandarte Island, annual variation in climate had little effect on population growth. In contrast, Sæther et al. (19) found that climate influenced the amount of winter habitat available to dippers (Cinclus cinclus), their rate of recruitment to populations, and their rate of population growth. Similar effects of climate on influential demographic rates have been observed in populations of swallows (18) and warblers (20). Overall, these results suggest that populations will vary in their response to climate change depending in part on the strength and mechanism of population regulation.
Acknowledgments
We thank J. N. M. Smith, J. M. Reid, K. Martin, S. E. Runyan, A. B. Marr, K. D. O'Connor, and an anonymous reviewer for helpful comments on this manuscript. Many field assistants helped in data collection over the years. Most recently these assistants included D. Dagenais, C. Saunders, R. Landucci, and A. K. Davis. The Tsawout and Tseycum First Nations bands kindly allowed us to work on Mandarte Island. Financial support for this research was provided by National Science Foundation Grant IBN 9458122 (to P.A.) and grants to S.W. from the Natural Sciences and Engineering Research Council and the Faculty of Forestry, University of British Columbia.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ENSO, El Niño Southern Oscillation; DDW, degree days of warming; SOI, Southern Oscillation index.
References
- 1.Hurrell, J. W. (1995) Science 269, 676-679. [DOI] [PubMed] [Google Scholar]
- 2.Allan, R., Lindesay, J. & Parker, D. (1996) in El Niño Southern Oscillation and Climate Variability (Commonwealth Scientific and Industrial Research Organization, Collingwood, Australia).
- 3.Stenseth, N. C., Mysterud, A., Ottersen, G., Hurrell, J. W., Chan, K. S. & Lima, M. (2002) Science 297, 1292-1296. [DOI] [PubMed] [Google Scholar]
- 4.Visbeck, M. H., Hurrell, J. W., Polvani, L. & Cullen, H. M. (2001) Proc. Natl. Acad. Sci. USA 98, 12876-12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myneni, R. B., Keeling, C. D., Tucker, C. J., Asrar, G. & Nemani, R. R. (1997) Nature 386, 698-702. [Google Scholar]
- 6.Post, E. & Stenseth, N. C. (1999) Ecology 80, 1322-1339. [Google Scholar]
- 7.Visser, M. E., van Noordwijk, A. J., Tinbergen, J. M. & Lessells, C. M. (1998) Proc. R. Soc. London Ser. B 265, 1867-1870. [Google Scholar]
- 8.Forchhammer, M. C., Post, E. & Stenseth, N. C. (1998) Nature 391, 29-30.9422504 [Google Scholar]
- 9.Crick, H. Q. P., Dudley, C., Glue, D. E. & Thomson, D. L. (1997) Nature 388, 526. [Google Scholar]
- 10.Brown, J. L., Li, S. H. & Bhagabati, N. (1999) Proc. Natl. Acad. Sci. USA 96, 5565-5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn, P. O. & Winkler, D. W. (1999) Proc. R. Soc. London Ser. B 266, 2487-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parmesan, C. & Yohe, G. (2003) Nature 421, 37-42. [DOI] [PubMed] [Google Scholar]
- 13.Root, T. L., Price, J. T., Hall, K. R., Schneider, S. H., Rosenzweig, C. & Pounds, J. A. (2003) Nature 421, 57-60. [DOI] [PubMed] [Google Scholar]
- 14.Arcese, P. & Smith, J. N. M. (1988) J. Anim. Ecol . 57, 119-136. [Google Scholar]
- 15.Newton, I. (1998) Population Limitation in Birds (Academic, London).
- 16.Källander, H. & Karlsson, J. (1993) Condor 95, 1031-1034. [Google Scholar]
- 17.Houghton, J. T., Meria Filho, L. G., Callender, B. & Harris, N., eds. (1995) The Science of Climate Change (Cambridge Univ. Press, Cambridge, U.K.).
- 18.Møller, A. P. (2002) J. Anim. Ecol. 71, 201-210. [Google Scholar]
- 19.Sæther, B. E., Tufto, J., Engen, S., Jerstad, K., Røstad, O. W. & Skåtan, J. E. (2000) Science 287, 854-856. [DOI] [PubMed] [Google Scholar]
- 20.Sillett, T. S., Holmes, R. T. & Sherry, T. W. (2000) Science 288, 2040-2042. [DOI] [PubMed] [Google Scholar]
- 21.Arcese, P., Smith, J. N. M., Hochachka, W. M., Rogers, C. M. & Ludwig, D. (1992) Ecology 73, 805-822. [Google Scholar]
- 22.Grant, P. R., Grant, B. R., Keller, L. F. & Petren, K. (2000) Ecology 81, 2442-2457. [Google Scholar]
- 23.Curry, R. L. & Grant, P. R. (1989) J. Anim. Ecol. 58, 441-463. [Google Scholar]
- 24.Lindsey, G. D., Pratt, T. K., Reynolds, M. H. & Jacobi, J. D. (1997) Wilson Bull. 109, 339-343. [Google Scholar]
- 25.Tompa, F. S. (1964) Acta Zool. Fenn. 109, 3-73. [Google Scholar]
- 26.Arcese, P. (1989) Anim. Behav. 38, 958-979. [Google Scholar]
- 27.Arcese, P., Sogge, M. K., Marr, A. & Patton, M. (2003) The Birds of North America, No. 704, eds. Poole, A., Stettenheim, P. & Gill, F. (Acad. Nat. Sci., Washington, DC).
- 28.Barber, R. T. & Chavez, F. P. (1983) Science 222, 1203-1210. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber, R. W. & Schreiber, E. A. (1984) Science 225, 713-716. [DOI] [PubMed] [Google Scholar]
- 30.Smith, J. N. M., Montgomerie, R. D., Taitt, M. J. & Yom-Tov, Y. (1980) Oecologia 47, 164-170. [DOI] [PubMed] [Google Scholar]
- 31.O'Connor, R. J. (1978) Ibis 120, 534-537. [Google Scholar]
- 32.Dhondt, A. A. & Eyckerman, R. (1979) Ibis 121, 329-331. [Google Scholar]
- 33.Smith, J. N. M, Taitt, M. J., Rogers, C. M., Arcese, P., Keller, L. F., Cassidy, A. L. E. V. & Hochachka, W. M. (1996) Ibis 138, 120-128. [Google Scholar]