Abstract
The dynamics of cellular immunity against pathogens, and its interaction with the human MHC system, is a key area for empirical research, both within individual hosts and in population genetic surveys. However, in contrast with humoral immunity, the dynamics of cellular immunity have not been modeled at the population level. Here, we address this lacuna with a model of recently observed dramatic invasions of cytotoxic T lymphocyte escape mutants in human influenza A. In particular, we offer an explanation for the rapid fixation of a HLA-B27 restricted cytotoxic T lymphocyte escape mutant on the nucleoprotein that emerged in the 1993–1994 season. We find that the dynamics within a single season of influenza do not provide a realistic description, but a model of the full annual dynamics can offer a possible explanation. Our model is deterministic for the winter epidemic, and stochastic for the summer period. An escape mutant that leads to a slightly longer infection in a small proportion of hosts has a substantial advantage through summer persistence. Furthermore, if a small number of founding cases are responsible for initiating each epidemic, then this effect of rapid mutant fixation is amplified.
Humoral immunity to influenza has been much studied both in individual hosts and at the population level. In particular, the gradual antigenic drift and dramatic pandemic shift in the structure of the immunogenic surface hemagglutinin and neuraminidase molecules is one of the best documented cases of rapid evolution in pathogen population dynamics (1, 2). The cellular arm of the immune response against influenza has also been studied in individual hosts or in vitro (3–5). A recent focus of this work has been on the cytotoxic T lymphocyte (CTL) response (6, 7). These influenza studies echo considerable general interest in the importance of CTL responses and their interaction with MHC variability in human and other populations. In particular, the within-host dynamics of CTL responses with the human immune system have been addressed extensively empirically and in theory for HIV and a number of other infections (8, 9). Recently, the population-level signature of CTL restriction has been detected for HIV (10). However, the dynamics of CTL–MHC interactions have not yet been modeled in any system at the population level. Here we derive simple models for the population dynamics of CTL escape mutants of the influenza virus. This is based on recent empirical observations of remarkably rapid fixation of CTL escape mutants in influenza A infections of human populations (6, 11).
In particular, extensive sequencing and analysis of the nucleoprotein gene has shown changes in CTL epitopes of circulating influenza in humans in The Netherlands and other countries (6, 11–13) (Table 1). When mutations do occur, they apparently reach fixation remarkably rapidly. In particular, an epitope on the viral nucleoprotein (NP383–391), associated with a human MHC allele HLA-B27, disappeared after 1993. A single amino acid mutation (R384G) at the anchor residue of the epitope was sufficient to stop the peptide from binding to its HLA molecule, effectively removing it as a possible CTL target (6). This influenza variant without the CTL target epitope has also appeared in other countries in recent years (12) (Table 1). Earlier isolates show a mutation at the same site that would also have led to removal of the CTL epitope (R384K), during the 1988–1989 (6) and 1971–1972 (13) seasons. In both earlier cases, the mutation did not persist and the previous type resumed circulation.
Table 1. HLA-B27-associated CTL escape mutants: Summary of frequencies of amino acid 384 on nucleoprotein.
| No. of amino acids at position 384 of NP
|
||||
|---|---|---|---|---|
| Year | R | K | G | Source |
| 1933-1968 | 11 | 0 | 0 | Shu (13) |
| 1971 | 0 | 1 | 0 | |
| 1972 | 4 | 1 | 0 | |
| 1973-1990 | 32 | 0 | 0 | |
| 1989-1990 | 46 | 13 | 0 | Voeten (6) |
| 1991-1992 | 16 | 0 | 0 | |
| 1992-1993 | 16 | 0 | 0 | |
| 1993-1994 | 0 | 0 | 56 | |
| 1998-1999 | 0 | 0 | 15 | |
| 1993 | 1 | 0 | 0 | Lindstrom (12) |
| 1994 | 0 | 0 | 1 | |
| 1995-1996 | 6 | 0 | 0 | |
| 1997 | 0 | 0 | 2 | |
This table summarizes the results from three different sources, giving the number of isolates with a particular amino acid at position 384 of the nucleoprotein gene. When R is present at this site, it is known to be an anchor residue of a HLA-B27 restricted CTL epitope. The epitope was completely ineffective in the isolates with either of the alternate amino acids (6).
At first sight, the rapid spread of a CTL escape mutation is surprising, given the HLA restriction of the epitope. In the Caucasian population, only ≈8% of people are HLA-B27 positive (6). This varies between ethnic groups, but generally the prevalence of HLA-B27 is around this mark, or slightly lower (14, 15). If the benefit of the escape mutants only conveys a potential advantage in ≈8% of hosts, how could the mutation spread to fixation so quickly? This question is the focus of the current paper.
We address the basic dynamic issues by using simple population models. The population-level consequences of a CTL escape mutation are not well understood, so the goal here is to provide the most parsimonious qualitative explanation of the observed dynamics.
The rest of the paper introduces two simple models. The first is for the winter influenza epidemic only. We explore two simple assumptions for the escape mutant's advantage: an infection takes longer to clear in some hosts, or infection is more severe and some hosts are more infectious. This model does not offer a satisfactory picture of mutant invasion. Either the time scale for mutant invasion is too long, or the escape mutant must be unrealistically infectious or persistent. The second system thus considers the full annual dynamics of influenza, including the phase between epidemic seasons. It shows that a longer infectious period can enable a rapid invasion, and a bottleneck in the chain of infection reinforces this effect.
Within-Season Dynamics
The Model. Influenza is responsible for an epidemic each winter in each hemisphere, and persists at low levels between epidemics. As a simple first approach, we focus on the dynamics of this winter epidemic by using a deterministic system (Table 2). We model two strains circulating in the population, one with and one without a particular HLA-B27 associated CTL epitope. The two strains are distinguishable in 8% of the host population, and indistinguishable in the other 92% of hosts.
Table 2. Within-season dynamics.
| Increased infectiousness* |
| Ṡ = -β(I1 + I2a + kI2b)S |
| İ1 = βI1S - νI1 |
| İ2a = β(I2a + kI2b)(1 - p)S - νI2a |
| İ2b = β(I2a + kI2b)pS - νI2b |
| Increased infection length† |
| Ṡ = -β(I1 + I2a + I2b)S |
| İ1 = βI1S - νI1 |
| İ2a = β(I2a + I2b)(1 - p)S - νI2a |
| İ2b = β(I2a + I2b)pS - νI2b/k |
| Increased infection length† |
| Ṡ = -β(I1 + I2a + I2b)S |
| İ1 = βI1S - νI1 |
| İ2a = β(I2a + I2b)(1 - p)S - νI2a |
| İ2b = β(I2a + I2b)pS - νI2b/k |
| Host variables (measured in proportions) |
| S-Have had neither strain |
| I1-Are infected with nonmutant |
| I2a-Are HLA-B27-negative and infected with mutant |
| I2b-Are HLA-B27-positive and infected with mutant |
| Parameters |
| ν-Recovery rate (5 days) |
| β-Transmission coefficient (R0 × ν) |
| R0-Reproduction ratio (20) |
| P-Proportion HLA-B27 positive (0.08) |
| The basic equations used to represent the epidemic phase. Both systems are the same when k = 1, and identical to the standard SIR model. |
The mutant strain is k times more infectious in hosts who have type HLA-B27
The mutant strain leads to a k times longer infection in hosts who have type HLA-B27
An important issue is the estimation of the reproduction ratio, R0, which measures the maximal rate of spread of influenza in humans. This is the key parameter in describing the spread of an infectious disease. It is well characterized for some infections (16–18) but is not so well described for influenza. We use R0 = 20 for the strain before CTL escape mutation, based on an argument given in the Appendix. It is disappointing that a better estimate for influenza R0 is currently unavailable; however, we stress that the following results are not qualitatively sensitive to the size of R0.
There are a number of plausible ways in which a CTL escape mutant might scale up, via its behavior in an individual host, to act at the population level. The simplest is that the escape mutant has a higher effective R0; this could occur by some combination of two basic mechanisms. First, the escape mutant could have a longer infectious period: the host takes longer to clear infection, because what would have been an immunodominant CTL epitope is unavailable. Second, the mutant might have higher infectiousness, perhaps a higher viral load in the infecting host.
We also need to set the number of susceptibles at the start of the season. In vaccinating with one influenza strain and experimentally challenging with another, the probability of infection increases with the number of years between isolation of those strains (19). Pease (20) gave a linear fit to this apparent loss of immunity with time of strain isolation (his figure 2). Combining this with host births and deaths, we assume that a proportion of ≈0.05 of all hosts lose immunity to circulating influenza between epidemics, because of a combination of influenza antigenic drift and host demographics. Assuming that the fluctuation in the number of susceptibles is small, we set the initial condition at the start of each season as a proportion (1/R0 + 0.05/2) of hosts are susceptible to influenza, the factor of two coming from basic epidemic theory.
Fig. 2.
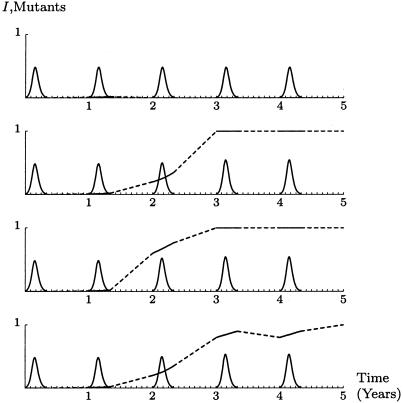
Multiseason dynamics. These are some realizations of the stochastic system. The solid curves are the number of infected expressed as a fraction of 1%. The mixed dashed and solid line is the proportion of infections that are caused by the mutant, with the dashed segment representing the change over the summer, including random founder choice with five founders. The mutant is introduced at autumn of year 1 at 1% relative prevalence. About 44% of runs are like the top example: the mutant does not survive until the second year. If the mutant does survive the first summer, then it tends to go to fixation rapidly.
Finally, for the nonmutant, we suppose an infectious period ν-1 = 5 days, (ref. 21 gives range of acute infection symptoms as 3–7 days).
Results. Both possibilities (longer infectious period or increased infectiousness of the mutant in 8% of hosts) could support rapid mutant invasion at extreme parameter values. However, as illustrated in Fig. 1, both required a very substantial advantage in HLA-B27 positive hosts.
Fig. 1.
Epidemic dynamics. (a and b) HLA-B27-positive hosts are twice as infectious when infected with the mutant. (c and d) HLA-B27-positive hosts have an infection of twice the normal duration when infected by the mutant (Table 2 with k = 2). The solid curves of a and c give the fraction of 1% of hosts infected (starting from 0.01%). The dashed curves give the proportion of these that are infected by the mutant. Initially, the mutant accounted for 10% of all infections. The solid curves of b and d represent the overall effect on the proportion of infected that are CTL escape mutants of the epidemic season. The dotted line is the identity (diagonal), for comparison.
Fig. 1 a and b shows one epidemic where the mutant is twice as infectious as the nonmutant in HLA-B27-positive hosts. An emerging mutant would take many years to invade, even with this substantial effect in 8% of hosts. Fig. 1 c and d shows an epidemic with the mutant infectious for twice the usual length of time in HLA-B27-positive hosts. Here an emerging mutant could invade over the course of several years, and the epidemics are only marginally larger than usual. It seems unlikely that this level of advantage is obtained in practice, because no increase of disease severity in particular hosts was reported, nor was the epidemic unusually large in the years that the mutant first appeared.
In summary, these simple models can show the escape mutant fixating at the population level. However, the models do not explain the rapid speeds of fixation. With these results in mind, further biological aspects of influenza dynamics must be considered, and an alternative model must be sought.
Annual Dynamics
The Model. A prolonged infectious period offers the mutant some advantage during an epidemic, although the required extension to the infectious period must be substantial (Fig. 1). The mutant advantage is most marked in the tail of the epidemic (Fig. 1c). Further, it is known that the lengths and distributions of infectious periods can also affect stochastic persistence in acute infections during epidemic troughs (22–24). This suggests that we consider how a longer infectious period (in some hosts) might affect mutant invasion when the full annual dynamics, including epidemic troughs, are taken into account.
Multiphase models have been developed previously (25), in particular for annual influenza with a single strain at any given time and a transition between epidemics (26). Here we present a model that includes the CTL escape mutant, and thus needs to consider dynamics between epidemics in more detail. The epidemic part of the year (winter) is the deterministic system given above. The 8 months of infections between epidemic seasons is treated as a stochastic system, details are given in the Appendix. A small number of founder infections initiate the next epidemic the following winter. In both phases, the mutant causes a slightly longer infection in 8% of hosts.
Results. The mutant gains substantial advantage in the time between epidemics. During epidemic troughs, increased persistence from its longer infectious period is key. Despite having only a sightly larger reproductive ratio (20.64 as opposed to 20), the mutant can invade rapidly.
Fig. 2 shows four different realizations of the system for the case when there are five founders. Around 44% of the time, the mutant does not survive the first summer. In other cases, the mutant usually reaches fixation after 2–3 years.
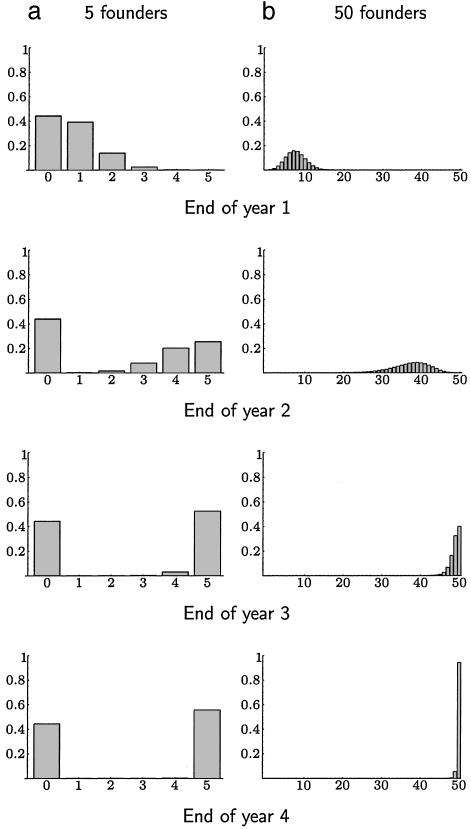
If the number of founders is small, this advantage can be amplified by chance in some years and lost in others. Fig. 3 compares the probability distribution of the number of mutants among the founders after the start of successive winters. The small number of founders gives the mutant a chance to reach fixation very rapidly, but a larger number of founders gives a greater probability of fixation in the long term. In the extreme case of a single infection sparking each epidemic, the mutant reaches fixation after one season 15% of the time, and otherwise vanishes.
Fig. 3.
Effect of different numbers of founders. A comparison of the probability distribution of the number of mutants at the end of each year for a small and large number of founders. The left column of graphs is for five founders, and the right column is for 50 founders. The mutant is introduced at the start of the first year, accounting for 1% of all infection. The rows correspond to the end of successive years. The x axis gives the number of the founders cases that are caused by the mutant, and the y axis gives the probability of this number. For five founders, the mutant reaches fixation with probability ≈56%. Typically, it has reached either near fixation or extinction after 2 years. For 50 founders, the mutant nearly always will eventually reach fixation, though may take longer than for five founders. In the extreme of a single founder, any mutant is always forced to fixation or goes extinct after the first summer.
We took the mutant infection to last 7 days, rather than 5, in 8% of hosts. Changing this to 8 days (not shown here) gives the mutant a much larger advantage in the summer. For a mutant infectious period of 6 days, there is still an advantage, but rapid fixation becomes more reliant on there being a small number of founders.
Discussion and Conclusions
The surface proteins of circulating influenza (hemagglutinin and neuraminidase) undergo continual mutation in response to strong immune selection pressure from the human population (1). In contrast, the evolutionary dynamics of CTL epitopes are more punctuated. Furthermore, when changes have occurred, they appear to fixate rapidly (6, 11). We have aimed to offer a qualitative description of this phenomenon with particular focus on the recent HLA-B27-associated escape mutant (6). There are several models considering CTL escape within a host, for example, HIV (refs. 9 and 27, but see ref. 10 for an empirical study at the population level). The present study models the population level impact of CTL escape and MHC restriction.
An initial model of the winter epidemic alone did not offer a sufficient mechanism for CTL escape fixation. By contrast, a model of the full seasonal dynamics provides a biologically plausible explanation for rapid fixation. The combination of two effects (summer stochastic persistence, autumn bottleneck) offers a possible mechanism for the rapid emergence and fixation of a CTL escape mutant. The mutant persists better between seasons through a longer infectious period in some hosts. The rapid emergence of escape in mutations that are observed can be explained by the compounding of this advantage with the “make or break” amplification effect of the epidemic-founding bottleneck.
It is interesting to note that the season before the fixation of the HLA-B27 epitope mutation (1992–1993) was dominated by influenza B, with a low prevalence of influenza A; this would greatly increase the potential of a bottleneck, effectively a “summer” that lasts for almost 2 years.
Biological Impact of CTL Escape in Influenza
A key assumption in our most successful model is that CTL escape mutant virus has a longer infectious period in the relevant hosts. This hypothesis should be testable via longitudinal studies of viral shedding in human or other host populations. Such studies would be demanding, but their results could have profound implications for our understanding of influenza dynamics. [Note also a very recent study where influenza CTL escape mutants caused longer infections in mice (7).]
Complications and Caveats
Model Structure and Complexity. The systems presented here are kept simple to explore influenza dynamics in as parsimonious a way as possible. However, we expect these mechanisms leading to rapid escape fixation (summer advantage, autumn bottleneck) to have direct parallels in more complex models. The models given in this paper can thus be thought of as possible components of a wider system. Their role here is to provide a minimal platform on which to explore and demonstrate our proposed mechanism of escape.
The Appendix gives an argument for the rough magnitude of R0, the reproduction ratio. Our results are not highly sensitive to R0, but to get more quantitatively realistic dynamics, better parameter estimates will be needed. In addition, we did not include spatial structure, whereas influenza has complex geographic patterns, particularly the alternating epidemics in the northern and southern hemispheres' winters (4). In a model including this global description, chains of infection cross between countries and hemispheres over the year, so the parallel to the autumn bottleneck will be complex, including both seasonal and spatial aspects.
The antigenic drift structure of influenza A was treated simply as a loss of host immunity with time. In practice, the interplay between humoral and cellular immunodynamics may be important. The theoretical structures needed to model influenza drift dynamics are being developed (2, 28), and an important future challenge is to combine drift and CTL escape dynamics in a population model.
Long-Term CTL Memory. Another possible explanation for mutant invasion would be if CTL immunity were important for long-term protection against influenza. However, this is not thought to be the case in practice (29). A model of escape from cross-protective immunity (not given here) shows that both mutant invasion and the size of the epidemic are highly sensitive to the relative importance of the epitope in a HLA-B27 positive host. If the CTL epitope is not very important for long-term immunity, then the mutant has little advantage. If the epitope is significant, then an immune escape leads to a massive epidemic across the population, which has not to date been detected in association with CTL escape. There is little middle ground between these extremes.
Side Effects of Mutation. The basic assumption here was that the escape mutant did not behave differently to the original strain, except for a single effect in 8% of hosts. This is likely to be a simplification. For example, compensatory mutations may be necessary, otherwise the virus may reproduce less well (30). If the escape mutant were slightly less fit, then it may be at an advantage while the epidemic was in decline and between seasons, but at a disadvantage when the winter epidemic is taking off. This might be explored, because a possible mechanism for the transient mutants that have been observed. Also, there are several CTL target epitopes on the nucleoprotein; in particular, there is an overlapping HLA-B08-restricted epitope.
More broadly, it is intriguing that the particularly immunodominant HLA-A2 restricted M158–66 epitope has remained stable since at least 1918, despite a frequency of type HLA-A2 of about one-quarter of the population (15). By our reasoning, we would expect a strong pressure favoring a spread of such a mutant. Note, however, that the viral sequence at this point may contribute vitally to the large-scale structure of the M1 protein, so that functional constraints may predominate here. Balancing structural constraints against immunological opportunities is a particularly interesting area for future work.
Impact of Other HLA Alleles. We assumed here that the escape mutant gains a net advantage in HLA-B27-positive people, and thus that HLA-B27-negative hosts have alternate target epitopes available. Another hypothesis would be that the original virus is at a disadvantage in HLA-B27-positive people and that the escape mutant loses this disadvantage. A simple model of this scenario (not shown) indicates that the above qualitative results still hold, although the effect is weaker, a stronger founder effect is required to drive rapid fixation. In practice, however, there are many CTL epitopes in influenza A with different HLA restrictions. Teasing out how the full complexity of host and pathogen genetics drives the spread of CTL mutants is an important area for future work (31). Influenza A provides a relatively tractable model for considering the general issue of population level impact of CTL responses.
Acknowledgments
We thank two anonymous referees for their comments and Janet Daly, Paul Digard, Eddie Holmes, and David Rand for helpful discussion. J.R.G. is supported financially by Queens' College, Cambridge, G.F.R. and A.D.M.E.O. are supported by European Union Grant QLRT-2001-1034 (Novaflu 2001), and B.T.G. is supported by the Wellcome Trust and the U.K. Biology and Biotechnology Research Council.
Appendix
The R0 of Influenza. The R0 of influenza is notoriously hard to estimate. Murray used a simple epidemic model to fit an outbreak in a boys boarding school in 1978 (32). His parameter fits suggest R0 = 3.8. However, the implied infectious period is ≈2.3 days, on average, which is unusually low for influenza. This could be because the boys were sent to the infirmary when they showed symptoms, effectively removing contact with susceptibles. Scaling up the infectious period to 5 days would place R0 at 8.3.
Spicer fitted a simple daily time-series model to weekly deaths from influenza and influenzal pneumonia in England and Wales and also separately to London for the epidemic season from a range of 1958–1973 (33, 34). This model was based on the more complex model for influenza in U.S.S.R. by Baroyan et al. (35, 36). Scaling up Spicer's estimates of R per day, times the expected infectious period gives R0 between 11.5–22.7. The top end of this range was from the epidemic of 1969–1970, which was shortly after a subtype shift so corresponds to a largely susceptible population.
The present paper uses R0 = 20, guided by Spicer's studies and the assumption that it is substantially larger than Murray's value for a single outbreak in nonnaïve hosts. It is disappointing that a more confident value could not be found and, clearly, further studies to establish the R0 of various influenza strains are needed.
The Annual Model. The epidemic phase is as in Table 2 and lasts for 4 months. After this period, the number of susceptibles is frozen at S = 1/R0 - 0.025. The next 8 months are modeled as a stochastic system. The branching process of infection can be described as a Markov chain:
 |
1 |
where ki is the probability that there are i infectious individuals.
Most chains of infection from the end of an epidemic will fade out over the summer, so we require the probability that a branch of infection initiated by a single infection persists through to the next epidemic. Define Pn(t) to be the probability that the chain of infection will fail by a time t in the future, given that there are currently n individuals infected, then
 |
2 |
All branches operate independently as the susceptible proportion does not vary, so Pn(t) = (P1(t))n, and this solution follows
 |
3 |
The mutant strain is more complex, but following similar lines:
 |
4 |
where m is the probability a chain of infection failing by time t, starting from an infection in one HLA-B27-negative host, and n is the corresponding probability from one HLA-B27-positive host.
The final values of these systems, found numerically, give the probability of a chain of infection spanning the 8 months between epidemic seasons. Taking the final values of the number of infecteds from the previous deterministic epidemic, and weighting them with these probabilities gives the distribution of mutant and nonmutant branches just before the following winter. A number of founder infecteds are drawn from this distribution, and the next epidemic is initiated with that proportion of escape mutants.
There are two approximations in this processes. First, we assume that the distribution of persisting branches corresponds to the distribution of infections at the end of the summer, and secondly, mutant infections are distributed in the host at the start of the epidemic independently of host HLA type. Both of these simplifying assumptions can be shown to weight against the escape mutant, but are small in effect.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: CTL, cytotoxic T lymphocyte.
References
- 1.Earn, D. J. D., Dushoff, J. & Levin, S. A. (2002) Trends Ecol. Evol. 17, 334-340. [Google Scholar]
- 2.Ferguson, N. M., Galvani, A. P. & Bush, R. M. (2003) Nature 422, 428-433. [DOI] [PubMed] [Google Scholar]
- 3.Benninck, J. R., Yewdell, J. W. & Gerhard, W. (1982) Nature 296, 75-76. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson, K. G., Webster, R. G. & Hay, A. J. (1998) Textbook of Influenza (Blackwell, Oxford).
- 5.McMichael, A. J., Michie, C. A., Gotch, F. M., Smith, G. L. & Moss, B. (1986) J. Gen. Virol. 67, 719-726. [DOI] [PubMed] [Google Scholar]
- 6.Voeten, J. T. M., Bestebroer, T. M., Nieuwkoop, N. J., Fouchier, R. A. M., Osterhaus, A. D. M. E. & Rimmelzwaan, G. F. (2000) J. Virol. 74, 6800-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voeten, J. T. M., Bestebroer, T. M., Nieuwkoop, N. J., Fouchier, R. A. M., Osterhaus, A. D. M. E. & Rimmelzwaan, G. F. (2000) J. Virol. 74, 6800-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMichael, A. J. (1998) Cell 93, 673-676. [DOI] [PubMed] [Google Scholar]
- 9.Burroughs, N. J & Rand, D. A. (1998) Proc. R. Soc. London Ser. B 265, 529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore, C. B., John, M., James, I. R., Christiansen, F. T., Witt, C. S. & Mallal, S. A. (2003) Science 296, 1439-1443. [DOI] [PubMed] [Google Scholar]
- 11.Boon, A. C. M., de Mutsert, G, Graus, Y. M. F., Fouchier, R. A. M., Sintnicolaas, K., Osterhaus, A. D. M. E. & Rimmelzwaan, G. F. (2002) J. Virol. 76, 2567-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindstrom, S. E., Hiromoto, Y., Nerome, R., Omoe, K., Sugita, S., Yamazaki, Y., Takahashi, T. & Nerome, K. (1998) J. Virol. 72, 8021-8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu, L. L., Bean, W. J. & Webster, R. G. (1993) J. Virol. 67, 2723-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan, M. A. (1995) Curr. Opin. Rheumatol. 7, 263-269. [DOI] [PubMed] [Google Scholar]
- 15.Marsh, S. G. E., Parham, P. & Barber, L. D. (2000) The HLA Factsbook (Academic, London).
- 16.Anderson, R. M & May, R. M. (1991) Infectious Diseases of Humans: Dynamics and Control (Oxford Science, Oxford).
- 17.Grenfell, B. T., Bjørnstad, O. N. & Finkenstädt, B. (2002) Ecol. Monogr. 72, 185-202. [Google Scholar]
- 18.Bjørnstad, O. N., Grenfell, B. T. & Finkenstädt, B. (2002) Ecol. Monogr. 72, 169-184. [Google Scholar]
- 19.Potter, C. W., Jennings, R, Nicholson, K, Tyrell, D. A. J. & Dickinson, K. G. (1977) J. Hyg. 79, 321-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pease, C. M. (1987) Theor. Popul. Biol. 31, 422-452. [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg, H. S. (1980) in Microbiology, eds. Davis, B. D., Dulbecco, R., Eisen, H. N. & Ginsberg, H. S. (Harper & Row, New York).
- 22.Keeling, M. J. & Grenfell, B. T. (1998) Math. Biosci. 147, 207-226. [DOI] [PubMed] [Google Scholar]
- 23.Rohani, P., Earn, D. J. & Grenfell, B. T. (1999) Science 286, 968-971. [DOI] [PubMed] [Google Scholar]
- 24.Keeling, M. J. & Grenfell, B. T. (2002) Proc. R. Soc. London Ser. B 269, 335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May, R. M. (1995) Am. Nat. 125, 573-584. [Google Scholar]
- 26.Andreasen, V. (2003) J. Math. Biol. 46, 504-536. [DOI] [PubMed] [Google Scholar]
- 27.Nowak, M. A., May, R. M., Phillips, R. E., Rowland-Jones, S., Lalloo, D. G., McAdam, S., Klenerman, P., Köppe, B., Sigmund, K., Bangham, C. R. M. & McMichael, A. J. (1995) Nature 375, 606-611. [DOI] [PubMed] [Google Scholar]
- 28.Gog, J. R. & Grenfell, B. T. (2002) Proc. Natl. Acad. Sci. USA 99, 17209-17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rimmelzwaan, G. F & Osterhaus, A. D. M. E. (1995) Vaccine 13, 703-705. [DOI] [PubMed] [Google Scholar]
- 30.Kelleher, A. D., Long, C., Holmes, E. C., Allen, R. L., Wilson, J., Conlon, C., Workman, C., Shaunak, S., Olson, K., Goulder, P., et al. (2001) J. Exp. Med. 193, 375-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boon, A. C. M., de Mutsert, G., Graus, Y. M. F., Fouchier, R. A. M., Sintnicolaas, K., Osterhaus, A. D. M. E. & Rimmelzwaan, G. F. (2002) J. Virol. 76, 582-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray, J. D. (1993) Mathematical Biology (Springer, New York).
- 33.Spicer, C. C. (1979) Br. Med. Bull. 35, 23-28. [DOI] [PubMed] [Google Scholar]
- 34.Spicer, C. C. (1982) in Influenza Models, ed. Selby, P. (MTP Press, Lancaster, U.K.), pp. 133-137.
- 35.Baroyan, O. V., Rvachev, L. A., Basilevsky, U. V., Ermakov, V. V., Frank, K. D., Rvachev, M. A. & Shashkov, V. A. (1971) Adv. Appl. Probab. 3, 224-226. [Google Scholar]
- 36.Rvachev, L. A. & Longini, I. M. (1985) Math. Biosci. 75, 3-22. [Google Scholar]