Abstract
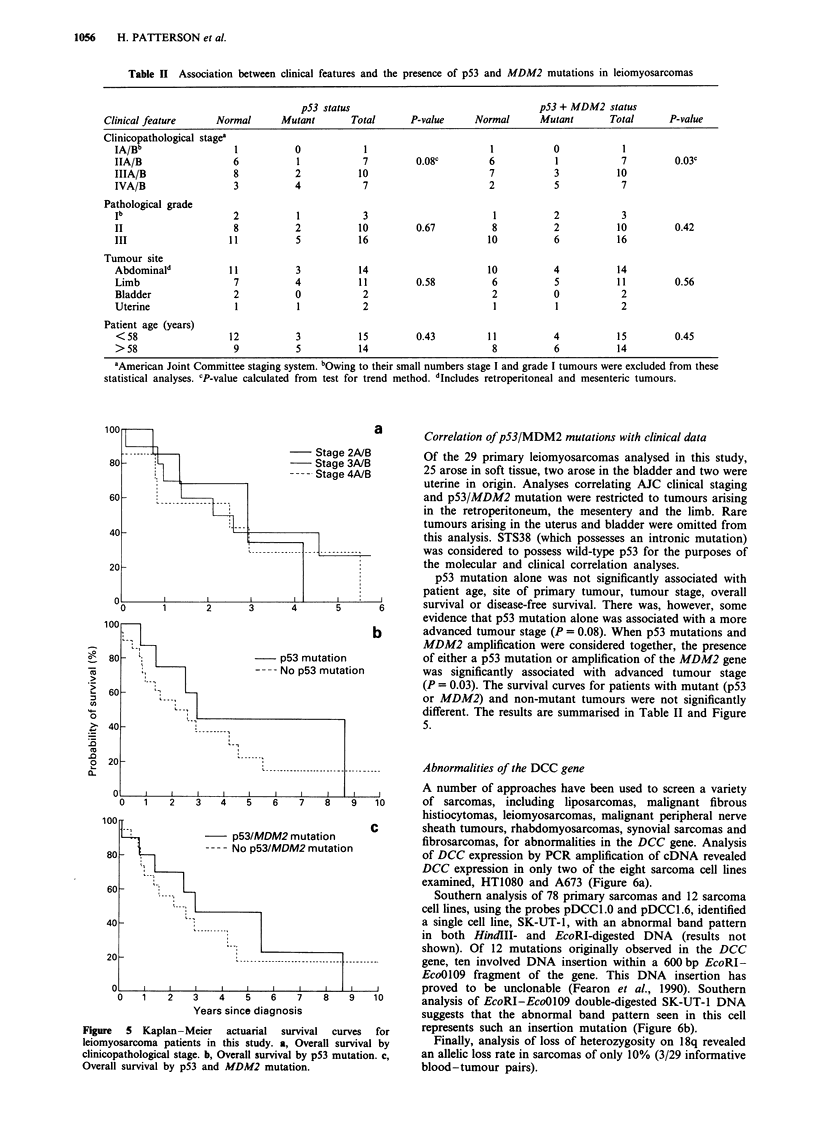
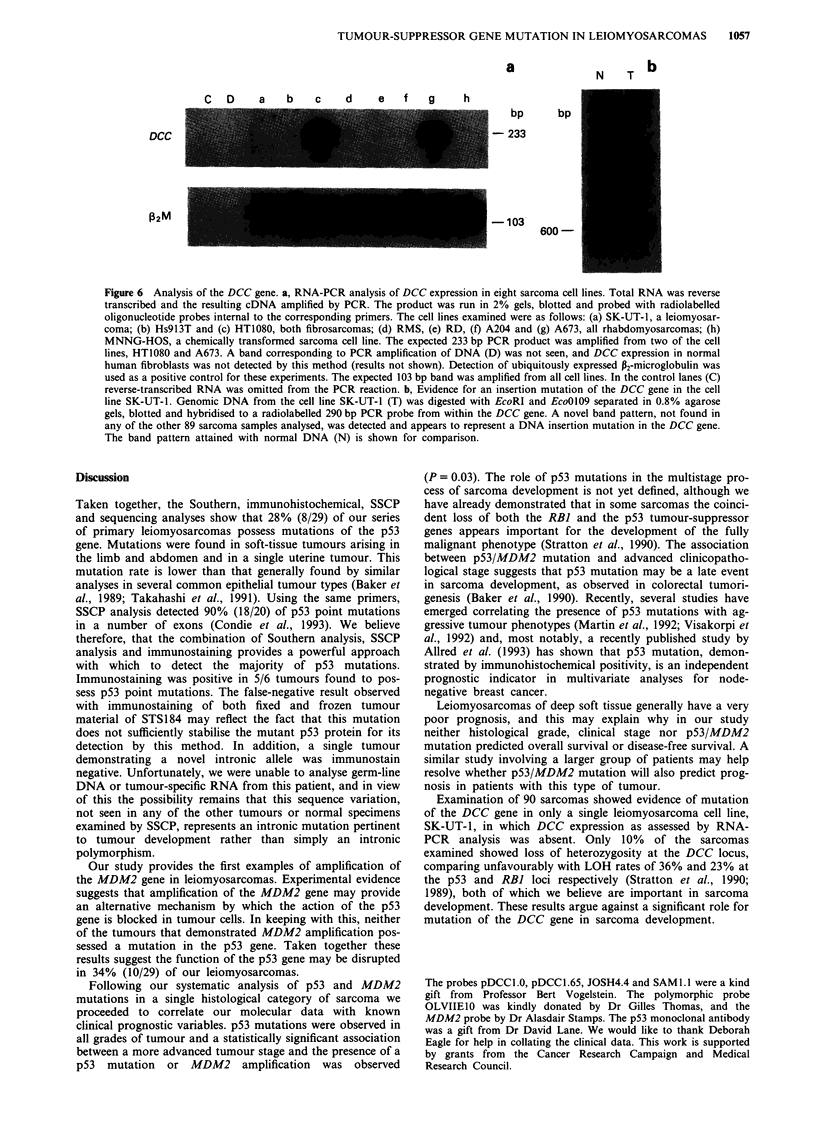
In this study we have screened a series of 29 primary leiomyosarcomas for abnormalities of both the p53 gene and the MDM2 gene, which encodes a p53-associated protein. SSCP (single-strand conformation polymorphism) analysis and direct sequencing of polymerase chain reaction (PCR)-amplified DNA were used to establish that 6/29 tumours possessed point mutations of the p53 gene. Using a monoclonal antibody that recognises the p53 protein in immunohistochemical staining experiments, we observed overexpression of the p53 protein in five of the six tumours containing point mutations in the p53 gene. Southern analysis of tumour DNA revealed that 2/29 tumours demonstrated amplification of the MDM2 gene. When considered together, these results indicate that alterations in both the p53 gene and MDM2 gene are important in the development of a significant minority of leiomyosarcomas. In addition, we have demonstrated a significant association between the presence of abnormalities of the p53 gene or MDM2 genes in leiomyosarcomas and a more advanced clinicopathological stage (P = 0.03). We have also examined the role of the DCC tumour-suppressor gene in the development of human soft-tissue tumours in a variety of histological types. Except for evidence of a rearrangement in a single leiomyosarcoma cell line, SK-UT-1, we have found no direct evidence to support a role for mutation of the gene in the development of human soft-tissue tumours.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. C., Clark G. M., Elledge R., Fuqua S. A., Brown R. W., Chamness G. C., Osborne C. K., McGuire W. L. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993 Feb 3;85(3):200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Preisinger A. C., Jessup J. M., Paraskeva C., Markowitz S., Willson J. K., Hamilton S., Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990 Dec 1;50(23):7717–7722. [PubMed] [Google Scholar]
- Bartek J., Iggo R., Gannon J., Lane D. P. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene. 1990 Jun;5(6):893–899. [PubMed] [Google Scholar]
- Chen P. L., Chen Y. M., Bookstein R., Lee W. H. Genetic mechanisms of tumor suppression by the human p53 gene. Science. 1990 Dec 14;250(4987):1576–1580. doi: 10.1126/science.2274789. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condie A., Eeles R., Borresen A. L., Coles C., Cooper C., Prosser J. Detection of point mutations in the p53 gene: comparison of single-strand conformation polymorphism, constant denaturant gel electrophoresis, and hydroxylamine and osmium tetroxide techniques. Hum Mutat. 1993;2(1):58–66. doi: 10.1002/humu.1380020111. [DOI] [PubMed] [Google Scholar]
- DeLeo A. B., Jay G., Appella E., Dubois G. C., Law L. W., Old L. J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A. 1979 May;76(5):2420–2424. doi: 10.1073/pnas.76.5.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliyahu D., Raz A., Gruss P., Givol D., Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984 Dec 13;312(5995):646–649. doi: 10.1038/312646a0. [DOI] [PubMed] [Google Scholar]
- Fakharzadeh S. S., Trusko S. P., George D. L. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 1991 Jun;10(6):1565–1569. doi: 10.1002/j.1460-2075.1991.tb07676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin A. J., Stanley W. S., Bennett D. D., Sullivan J. L., Sens D. A. The in vitro growth, heterotransplantation, and differentiation of a human rhabdomyosarcoma cell line. Am J Pathol. 1986 Oct;125(1):208–217. [PMC free article] [PubMed] [Google Scholar]
- Gusterson B. A., Anbazhagan R., Warren W., Midgely C., Lane D. P., O'Hare M., Stamps A., Carter R., Jayatilake H. Expression of p53 in premalignant and malignant squamous epithelium. Oncogene. 1991 Oct;6(10):1785–1789. [PubMed] [Google Scholar]
- Hinds P., Finlay C., Levine A. J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989 Feb;63(2):739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Currie G. A. Cellular immortalization by a cDNA clone encoding the transformation-associated phosphoprotein p53. Nature. 1984 Dec 13;312(5995):651–654. doi: 10.1038/312651a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Marlhens F., Delattre O., Bernard A., Olschwang S., Dutrillaux B., Thomas G. RFLP identified by the anonymous DNA segment OL VII E10 at 18q21.3 (HGM no. D18S8). Nucleic Acids Res. 1987 Feb 11;15(3):1348–1348. doi: 10.1093/nar/15.3.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. M., Filipe M. I., Morris R. W., Lane D. P., Silvestre F. p53 expression and prognosis in gastric carcinoma. Int J Cancer. 1992 Apr 1;50(6):859–862. doi: 10.1002/ijc.2910500604. [DOI] [PubMed] [Google Scholar]
- Momand J., Zambetti G. P., Olson D. C., George D., Levine A. J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992 Jun 26;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Mowat M., Cheng A., Kimura N., Bernstein A., Benchimol S. Rearrangements of the cellular p53 gene in erythroleukaemic cells transformed by Friend virus. Nature. 1985 Apr 18;314(6012):633–636. doi: 10.1038/314633a0. [DOI] [PubMed] [Google Scholar]
- Narayanan R., Lawlor K. G., Schaapveld R. Q., Cho K. R., Vogelstein B., Bui-Vinh Tran P., Osborne M. P., Telang N. T. Antisense RNA to the putative tumor-suppressor gene DCC transforms Rat-1 fibroblasts. Oncogene. 1992 Mar;7(3):553–561. [PubMed] [Google Scholar]
- Noonan K. E., Roninson I. B. mRNA phenotyping by enzymatic amplification of randomly primed cDNA. Nucleic Acids Res. 1988 Nov 11;16(21):10366–10366. doi: 10.1093/nar/16.21.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
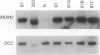
- Oliner J. D., Kinzler K. W., Meltzer P. S., George D. L., Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992 Jul 2;358(6381):80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Prosser J., Thompson A. M., Cranston G., Evans H. J. Evidence that p53 behaves as a tumour suppressor gene in sporadic breast tumours. Oncogene. 1990 Oct;5(10):1573–1579. [PubMed] [Google Scholar]
- Rhim J. S., Park D. K., Arnstein P., Huebner R. J., Weisburger E. K., Nelson-Rees W. A. Transformation of human cells in culture by N-methyl-N'-nitro-N-nitrosoguanidine. Nature. 1975 Aug 28;256(5520):751–753. doi: 10.1038/256751a0. [DOI] [PubMed] [Google Scholar]
- Rodrigues N. R., Rowan A., Smith M. E., Kerr I. B., Bodmer W. F., Gannon J. V., Lane D. P. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. O., Cohen J., Enzinger F., Hajdu S. I., Heise H., Martin R. G., Meissner W., Miller W. T., Schmitz R. L., Suit H. D. A clinical and pathological staging system for soft tissue sarcomas. Cancer. 1977 Oct;40(4):1562–1570. doi: 10.1002/1097-0142(197710)40:4<1562::aid-cncr2820400428>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Soussi T., Caron de Fromentel C., May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene. 1990 Jul;5(7):945–952. [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
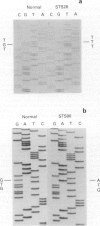
- Stratton M. R., Moss S., Warren W., Patterson H., Clark J., Fisher C., Fletcher C. D., Ball A., Thomas M., Gusterson B. A. Mutation of the p53 gene in human soft tissue sarcomas: association with abnormalities of the RB1 gene. Oncogene. 1990 Sep;5(9):1297–1301. [PubMed] [Google Scholar]
- Stratton M. R., Williams S., Fisher C., Ball A., Westbury G., Gusterson B. A., Fletcher C. D., Knight J. C., Fung Y. K., Reeves B. R. Structural alterations of the RB1 gene in human soft tissue tumours. Br J Cancer. 1989 Aug;60(2):202–205. doi: 10.1038/bjc.1989.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Takahashi T., Suzuki H., Hida T., Sekido Y., Ariyoshi Y., Ueda R. The p53 gene is very frequently mutated in small-cell lung cancer with a distinct nucleotide substitution pattern. Oncogene. 1991 Oct;6(10):1775–1778. [PubMed] [Google Scholar]
- Visakorpi T., Kallioniemi O. P., Heikkinen A., Koivula T., Isola J. Small subgroup of aggressive, highly proliferative prostatic carcinomas defined by p53 accumulation. J Natl Cancer Inst. 1992 Jun 3;84(11):883–887. doi: 10.1093/jnci/84.11.883. [DOI] [PubMed] [Google Scholar]