Abstract
Considerable evidence exists to support an association between psychological states and immune function. However, the mechanisms by which such states are instantiated in the brain and influence the immune system are poorly understood. The present study investigated relations among physiological measures of affective style, psychological well being, and immune function. Negative and positive affect were elicited by using an autobiographical writing task. Electroencephalography and affect-modulated eye-blink startle were used to measure trait and state negative affect. Participants were vaccinated for influenza, and antibody titers after the vaccine were assayed to provide an in vivo measure of immune function. Higher levels of right-prefrontal electroencephalographic activation and greater magnitude of the startle reflex reliably predicted poorer immune response. These data support the hypothesis that individuals characterized by a more negative affective style mount a weaker immune response and therefore may be at greater risk for illness than those with a more positive affective style.
Although considerable progress has been made in uncovering the molecular processes linking stress to suppressed immune function (1, 2), little is known about the systems-level processes underlying the relations between negative affect and physical health. Endocrine and immune products such as cortisol and proinflammatory cytokines have been implicated in the deleterious health consequences of psychosocial stress and negative affect. Therefore, it is plausible that the etiology of the dysregulation of these molecules likely involves the central circuitry governing the processing of emotional information. Of particular interest is the prefrontal cortex (PFC) because of its posited role in depression and associations with in vitro measures of immune function. The present study was designed to examine the relations between individual differences in psychophysiological indices of affective style and in vivo immune function. Affective style refers to individual differences in valence-specific features of emotional reactivity and affect regulation. Among its parameters are the magnitude and duration of emotion as well as the efficacy of processes invoked to regulate emotion (3, 4).
An impressive corpus of research has documented the impact of psychological stress on immune function. For example, caring for a loved one with dementia has been associated with an attenuated immune response to influenza vaccination (5, 6), slower wound healing (7), and increases in proinflammatory cytokines (8). Susceptibility to respiratory illness after virus exposure has been shown to increase with psychological stress in a dose-dependent way (9). Moreover, immune function has been shown to vary according to individual differences in reactivity to stress. One such study showed that cytotoxic T cell reactivity was associated with increases in anxiety to a naturalistic speech stressor (10). It is important to note that these studies demonstrate the role of stress in actual health outcome as well as in changes in immune parameters with potential implications for future health consequences. These health outcomes become especially salient when considering an aging population, one in which immune function is on a downward trajectory and influenza-related and respiratory illnesses are a leading cause of death.
Missing in these previous efforts to link stress and emotion to immune function outcomes has been measures of the brain. There is evidence to suggest that the manner in which individuals appraise an emotional situation, based on a questionnaire measure, accounts for significant variance in response to a viral challenge (11). The fact that individual differences in perceived stress accounted for variance in susceptibility to a viral challenge suggests that affective style is an important mediator of the impact of emotional challenges on immune function. Thus, the search for linkages between neural substrates of affective style, a more direct measure of such individual differences, and immunological parameters is warranted. Asymmetry in the activation of select territories of the PFC, assessed with measures of brain electrical activity, has been consistently related to individual differences in the tendency to experience negative affect. Individuals with high levels of right-sided prefrontal activation at baseline exhibit higher levels of dispositional negative affect and also show increased reactivity to acute negative affective challenges (see ref. 4 for review). For example, adults selected to show extreme levels of baseline right-prefrontal activation report greater dispositional negative affect than their left-frontally activated counterparts (12). In addition, adults with greater right-sided prefrontal activation at baseline also report more negative affect during an emotion-eliciting film clip, whereas those with greater left-sided prefrontal activation report more positive affect during a positive emotion-eliciting clip (13). In response to maternal separation, infants with right-sided prefrontal activation were more likely to cry compared with their left-activated counterparts (14). Of particular interest, previous studies have linked right-prefrontal activation to both poorer natural killer cell activity (15) and a larger decrement in natural killer cell activity in vitro after an emotional stressor (16).
Individuals with major depression are more likely to display greater relative right-sided prefrontal activation compared with their nondepressed counterparts (17), a pattern that is also present among remitted depressed patients who were euthymic at the time of testing (18). Depression has been linked with increased morbidity and mortality (for review see ref. 19) as well as with markers of decreased immunocompetence (for review see ref. 20). For example, depression has been associated with increased risk of cardiovascular and autoimmune disease (21, 22). Production of proinflammatory cytokines, thought to be a mediator of this effect, is increased in depression (23). The importance of the regulation of proinflammatory cytokines is accentuated in aging. Increases in IL-6, a potent proinflammatory cytokine, slow injury recovery rates and predict disability associated with old age as well as cognitive decline (24, 25). Irwin et al. (26) found that cellular immunity to herpes zoster virus, a virus that most of us are able to suppress easily, is compromised in major depression to a level resembling that seen in old age. In addition, subclinical negative emotion, indicative of a negative affective style, has been associated with decrements in immune function such as a smaller antibody response to vaccination with hepatitis B virus (27).
We investigated the relations between central and peripheral physiological measures that reflect individual differences in both dispositional affect and affective reactivity and immune function. In vivo immune function was measured by using an influenza vaccine challenge to assess individual differences in antibody titers in response to vaccine. Specifically, we examined the extent to which individual differences in brain electrical measures of activation asymmetry (at baseline and in response to an emotion-inducing task) and magnitude of emotion-modulated startle predict antibody titer rise in response to influenza vaccination.
Methods
Participants. Fifty-two individuals (24 females, ages 57–60 years) were recruited from the Wisconsin Longitudinal Study, a long-term study of a random sample of 10,317 graduates of Wisconsin high schools in 1957 (see http://dpls.dacc.wisc.edu/wls/index.html for more information). Participants determined to be left-handed or ambidextrous (28) were excluded (n = 12) from all analyses involving electroencephalograph (EEG) asymmetry because of evidence suggesting more complex patterns of lateralization in nonrighted individuals (e.g., ref. 29). Left-handed participants were retained in analyses examining emotion-modulated startle. All participants provided written informed consent as required by the University of Wisconsin (Madison) Human Subjects Committee.
Procedures. Emotion-induction task. Physiological measures were obtained under resting baseline and affect-induction conditions. For the affect-induction task, participants recalled an extremely positive and an extremely negative emotional experience. For the positive event, they were asked to recall an event during which they experienced intense happiness or joy, specifically the best time or experience in their life. For the negative event, they were asked to recall an event during which they experienced the most intense sadness, fear, or anger, the worst time or experience in their life. For both events they were asked to recall an event in which the experience of the emotion was relatively pure, i.e., not a mixed emotion or “bittersweet” moment. Participants were asked to think about the event and focus on the emotion experienced for 1 min while physiological measures were obtained. Next, they wrote about the event for 5 min, during which time no physiological measures were obtained (because of motion artifact). Finally, participants sat quietly for an additional 3 min while physiological measures were collected.
EEG measurement and quantification. Eight 1-min trials (four with eyes open and four with eyes closed) were recorded by using a modified Lycra electrode cap (Electro-Cap, Eaton, OH) positioned according to known cranial landmarks for the International 10-20 System according to a well established protocol (30). Twenty-nine EEG sites were recorded and referenced to physically linked ears. Impedances of all electrodes were <5,000 Ω, and the homologous ear reference sites were matched to within 500 Ω. Electrooculograms were recorded for the removal of eye-movement artifact: horizontal electrooculograms from the external canthi of each eye and vertical electrooculograms from the supra to suborbit of one eye. The recordings were edited to remove artifact caused by eye or muscle movement. A fast Hartley transform was applied to all 1.024-s artifact-free chunks of data, with a 50% overlap between chunks. Power density in the 8- to 13-Hz alpha band in μV2/Hz was computed and then averaged across the four trials within each condition and weighted by the number of artifact-free chunks to compute weighted means. A simple average was computed across condition (eyes open and eyes closed) and log-transformed to create the average power density across all eight trials (see refs. 12 and 31 for EEG procedural details). Asymmetry scores (log right–log left alpha power) were computed for each pair of homologous electrodes. Data were rereferenced to a whole-head average offline. In addition to the baseline recordings, an EEG was recorded for 1 min preceding and 3 min after the affective task described above. An average of all 1.024-s artifact-free chunks of data was computed for this recording period.
Startle measurement. Affect-modulated startle eye-blink magnitude was measured from silver–silver chloride electromyographic electrodes placed below the eye on the orbicularis oculi muscle in response to an acoustic startle probe (50-ms white-noise burst at 95 dB with a near-instantaneous rise time) as described by Jackson et al. (32). Startle eye-blink magnitude was calculated by subtracting the amount of integrated electromyographic activity at reflex onset from the maximum amount of integrated electromyographic activity between 20 and 120 ms after the probe onset. Noise-free trials with no perceptible eye-blink reflex were assigned a magnitude of zero and included in analyses. Blink magnitudes were z-transformed within subjects to control for large individual differences in response amplitude and baseline electromyographic levels. Nine probes with a mean interprobe interval of 20 s were presented during each 3-min period immediately after the affective task described above. Mean blink magnitudes were computed for each condition (two discernable responses per condition were necessary for inclusion in analyses). The duration of the prewriting period was insufficiently long to obtain a reliable startle measurement. Difference scores were calculated by subtracting mean startle magnitude during the positive postwriting period from that of the negative postwriting period to represent reactivity to the negative relative to positive affective task.
Immune-response measurement. Prevaccination serum samples were collected before vaccination with influenza virus vaccine trivalent types A and B (A/Beijing/262/95, A/Sydney/05/97, and B/Harbin/07/94; Pasteur Merieux Connaught, Paris). Two, 4, and 26 weeks (6 months) later, follow-up serum samples were collected and stored at -80°C until assayed. Antibody titer was determined by using the hemagglutination inhibition assay as described by the Centers for Disease Control (33). The resulting titer was log2-transformed.
Results
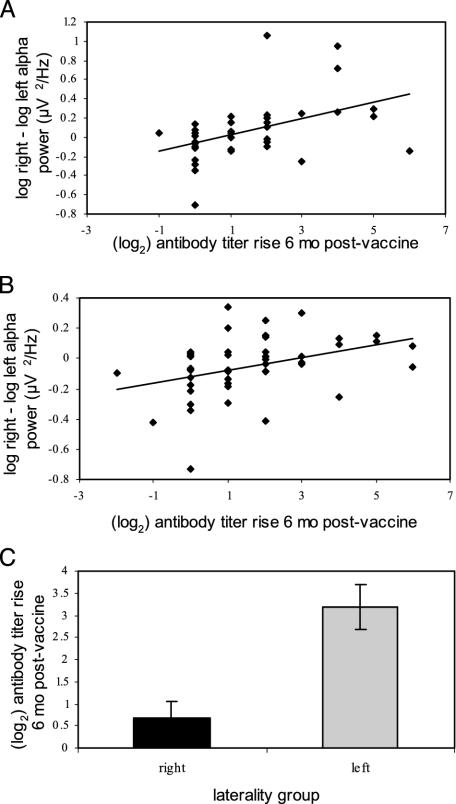
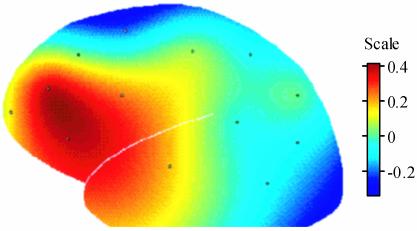
Baseline EEG Analyses. Pearson correlations were computed between baseline prefrontal activation asymmetry (FPF1/2, F7/8, F3/4, and FC3/4) and antibody titer rise at 6 months postvaccine. (Only results from the 6-month rise are reported here; those from the 2- and 4-week rise were consistent and significant although they had smaller effects than at 6 months.) Fig. 1 shows representative significant correlations found after correcting for multiple comparisons by using the Bonferroni method (adjusted α = 0.01) for baseline activation asymmetry at frontal pole (FPF2-FPF1; r = 0.37, P = 0.006) and lateral frontal (F8-F7; r = 0.38, P = 0.008) sites. Those individuals displaying greater relative right-prefrontal activation at baseline produced a smaller antibody rise. This relationship was also examined by selecting groups at the extreme ends of activation asymmetry, comprised of individuals in the top and bottom 25th percentile of prefrontal asymmetry. Individuals at the right-frontal extreme showed a significantly smaller rise in antibody titer than those at the left-frontal extreme [see Fig. 1; t(22) = -4.1, P < 0.01]. Fig. 2 highlights the fact that significant correlations were restricted to the anterior scalp regions (r values ranged from 0.19 to -0.1, and all P values > 0.19 in nonfrontal sites). Further, the correlation between frontal pole asymmetry and antibody titer rise was significantly different from a control site on the posterior scalp [PO3/4; t(49) = -2.24, P = 0.03] and antibody titer rise.
Fig. 1.
Scatter plots of baseline activation asymmetry in the frontal pole (A) and lateral frontal (B) scalp regions (right - left alpha power in log μV2/Hz) and antibody titer rise (log2) to influenza vaccine 6 months postvaccine. Note that higher numbers are associated with greater relative left-sided prefrontal activation. Subjects exhibiting more relative left-sided prefrontal activation both at the frontal pole (A: r = 0.37, P < 0.01, n = 52) and lateral frontal (B: r = 0.38, P < 0.01, n = 47) sites have a larger antibody titer response. (C) Bar graph of the mean antibody titer rise (log2) to influenza vaccine 6 months postvaccine for groups at the extreme ends of activation asymmetry [t(22) = -4.1, P < 0.01], comprised of individuals in the top and bottom 25th percentiles of asymmetry at the lateral frontal (F7/8) site.
Fig. 2.
Topographical spline-interpolated map of correlation coefficients across the scalp for baseline activation asymmetry (right - left alpha power) and antibody titer rise 6 months postvaccine, demonstrating that the strong positive associations are restricted to brain electrical measures recorded from frontal sites. Positive associations denote that greater relative left-sided activation is associated with higher antibody rise.
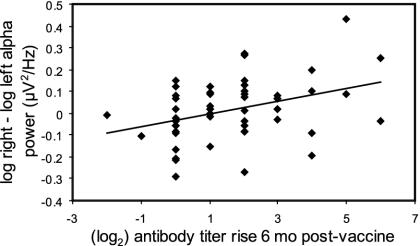
Affect-Induction Analyses. Pearson correlations were also computed for EEG asymmetry (FPF1/2, F7/8, F3/4, and FC3/4) during the 1- and 3-min recording periods flanking the affective writing tasks and antibody titer rise at 6 months postvaccine. Right-prefrontal activation preceding the negative affective task showed the same pattern of association with antibody titer rise at the frontal pole as it did during the baseline recording (r = 0.45, P = 0.006; see Fig. 3). Likewise, this correlation was significantly different from the correlation at a control site on the posterior scalp [PO3/4; t(32) = -2.44, P = 0.02]. However, this association was not observed at the lateral frontal site (r = 0.12, P = 0.50).
Fig. 3.
Scatter plot showing EEG activation asymmetry in the frontal polar scalp region (right - left alpha power in log μV2/Hz) during the negative affect induction and antibody titer rise (log2) to influenza vaccine 6 months postvaccine. Subjects exhibiting a more left-sided activation asymmetry at the frontal pole (r = 0.45, P < 0.01, n = 36) site have a larger antibody titer response.
The relation between frontal EEG asymmetry recorded during the 3 min after the negative affective task and antibody titer rise was consistent with but weaker than that found with the recording preceding the affective task (FPF1/2: r = 0.31, P > 0.01; F7/8: r = 0.26, P > 0.01). No significant relationships were found between frontal EEG asymmetry collected before or after the positive affective task and immune response. To rule out the contribution of prevaccine baseline antibody titers accounting for variance in the relation between prefrontal asymmetry and antibody increase in response to vaccine, correlations were examined between both baseline asymmetry and activation asymmetry in response to the negative writing challenge and baseline antibody titers. These analyses failed to reveal any significant associations.
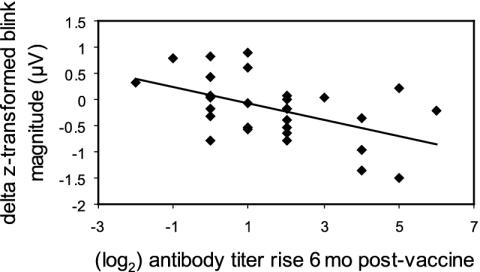
Emotion-Modulated Startle Analyses. A Pearson correlation was computed between relative startle reactivity to the affective tasks and antibody rise from prevaccine to 6 months postvaccine (see Fig. 4). A significant inverse correlation was found between startle magnitude in response to the negative, relative to the positive, affect induction and antibody titer rise (r = -0.50, P = 0.006), indicating that subjects showing increased startle magnitude after the negative compared with positive inductions had a smaller antibody rise. Correlations between frontal EEG asymmetry and startle reactivity were nonsignificant (r values ranged between 0 and -0.14; all P values > 0.45). As was done for the EEG analyses, we computed the correlation between startle reactivity and prevaccine baseline antibody titers to rule out variation in baseline titers as accounting for this relation. We found that the correlation was not significant.
Fig. 4.
Scatter plot showing the difference in eye-blink startle reflex magnitude (orbicularis oculi electroencephalograph, μV, z-transformed difference scores) during negative–positive affective induction and immune response to influenza vaccine. Subjects who showed a larger startle response during the negative relative to the positive affect induction showed a smaller antibody response to influenza vaccination (r = -0.50, P < 0.01, n = 29).
Regression Analyses. Baseline frontal pole EEG activation asymmetry and startle reactivity during the negative versus positive affective task were regressed on antibody titer rise from prevaccine to 6 months postvaccine. Startle-blink magnitude accounted for a significant portion of variance after that accounted for by prefrontal activation asymmetry was removed [FPF1/2: ΔR2 = 0.25, F(1,21) = 7.5, P = 0.01, partial r = -0.51; F7/8: ΔR2 = 0.19, F(1,19) = 5.66, P = 0.03, partial r = -0.48]. Together, prefrontal activation asymmetry and startle-blink magnitude accounted for >30% of the variance in antibody titer rise with either frontal EEG site [FPF1/2: multiple R2 = 0.31, F(2,21) = 4.6, P = 0.02; F7/8: multiple R2 = 0.36, F(2,19) = 5.3, P = 0.02].
Discussion
In the current study, three different physiological indicators of negative affective style predicted weaker antibody response to influenza vaccination: greater relative right-prefrontal EEG activation at baseline and greater relative right-prefrontal EEG activation and larger relative eye-blink startle magnitude in response to the negative affect induction.
These results replicate and extend previous in vitro work reported by Kang et al. (15) and Davidson et al. (16) in which lower natural killer cell activity was associated with greater relative right-sided prefrontal activation. In vivo measures allow us to probe the function of an intact immune system as it interacts with the nervous and endocrine systems to provide protection. In addition, these results expand the scope of the clinical literature addressing the links between depression and immunity to include central and peripheral physiological predictors of negative affect.
Although the specific pathways responsible for the association between negative affect and immune function cannot be determined in this study, data exist that suggest bidirectional communication between the PFC and certain immune cells. For example, IL-1, IL-2, and IL-6 alter dopaminergic activity in the PFC (34), and IL-1β increases PFC monoaminergic activity in response to mild stress (35). In turn, PFC dopaminergic function is altered in response to stress, and this alteration has been found to be asymmetric (36). Asymmetric disruptions of neocortical function via unilateral lesions have been found to differentially affect immune function with enhancement and suppression, respectively, of several indicators of immune function found in mice with partial right and left neocortical lesions (37). Vlajković et al. (38) showed a similar pattern when examining immune response in right and left cortical-lesioned rats.
In addition to the data suggesting interaction between the PFC and immune system, there are widespread connections to the immune system via the hypothalamic–pituitary–adrenal (HPA) axis, hippocampus, and amygdala (39). Functional manipulation of these regions has been shown to impact immune function. For example, Nisticó et al. (40) report an increase in the proliferative response of splenocytes to mitogen stimulation after infusion of a dopamine D1 agonist into the amygdala, whereas infusion into the CA1 region of the hippocampus resulted in a decreased response. Further, Vedhara et al. (5) report an inverse relation between mean basal salivary cortisol, an indicator of HPA-axis activation, and immune response to influenza vaccination. Our laboratory previously reported on associations between HPA-axis function and asymmetric prefrontal activity in nonhuman primates (e.g., ref. 41) and human infants (42), with animals and humans showing greater right-sided activation exhibiting higher levels of basal cortisol compared with their left-activated counterparts. Moreover, recent data from rhesus monkeys indicate that animals with higher levels of right-sided frontal activation also exhibit higher levels of cerebrospinal fluid measures of corticotrophin-releasing hormone, the molecule that initiates the cascade of changes in the HPA axis that culminate in release of cortisol (43).
Interestingly, the physiological measures of affective style, prefrontal activation asymmetry and emotion-modulated startle, themselves were not significantly correlated in this study. In a recent study from our laboratory, Jackson et al. (44) examined the time course of the emotion-modulated startle response and its relation to baseline frontal EEG asymmetry. We found that frontal EEG asymmetry was unrelated to startle magnitude probed during stimulus presentation but predicted a large portion of the variance in startle magnitude in response to a probe presented several seconds after stimulus offset. The startle-magnitude metric reported here reflects the response to the negative relative to the positive affect induction averaged across a 3-min recording. Unlike our previous paradigm that used pictures to induce a very brief and mild emotional response, the present study used an autobiographical writing task that produced a considerably more intense and longer-duration change in emotional state. Furthermore, the results of the regression analysis suggest that frontal EEG asymmetry and startle magnitude partially account for separate sources of variance in antibody titer rise. These measures likely reflect different central routes that influence a final common pathway, perhaps hypothalamic activity, which then modulates immune function.
The findings from this study provide evidence suggesting relations between central and peripheral indices of negative affective style and in vivo immune function. Future studies must now address the mechanisms by which such associations occur and the causal influence of the patterns of central activation on the immune responses. This now can be studied by using the lesion method in the rhesus monkey model and in humans by using transcranial magnetic stimulation to alter prefrontal activation patterns (45).
Acknowledgments
This research was supported by National Institute of Mental Health (NIMH) Grants MH43454, MH40747, P50-MH52354, and P50-MH61083, National Institute on Aging Grant P50-AG21079, NIMH Training Grant T32-MH18931, and the John D. and Catherine T. MacArthur Foundation Research Network on Mind–Body Interaction.
Abbreviations: PFC, prefrontal cortex; EEG, electroencephalograph.
References
- 1.McEwen, B. S. (2000) Brain Res. 886, 172-189. [DOI] [PubMed] [Google Scholar]
- 2.McEwen, B. S. (2002) Neurobiol. Aging 23, 921-939. [DOI] [PubMed] [Google Scholar]
- 3.Davidson, R. J. (1998) Cogn. Emotion 12, 307-330. [Google Scholar]
- 4.Davidson, R. J., Jackson, D. C. & Kalin, N. H. (2000) Psychol. Bull. 126, 890-909. [DOI] [PubMed] [Google Scholar]
- 5.Vedhara, K., Cox, N. K., Wilcock, G. K., Perks, P., Hunt, M., Anderson, S., Lightman, S. L. & Shanks, N. M. (1999) Lancet 353, 1969-1970. [DOI] [PubMed] [Google Scholar]
- 6.Kiecolt-Glaser, J. K., Glaser, R., Gravenstein, S., Malarkey, W. B. & Sheridan, J. (1996) Proc. Natl. Acad. Sci. USA 93, 3043-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiecolt-Glaser, J. K., Marucha, P. T., Malarkey, W. B., Mercado, A. M. & Glaser, R. (1995) Lancet 346, 1194-1196. [DOI] [PubMed] [Google Scholar]
- 8.Kiecolt-Glaser, J. K., Preacher, K. J., MacCallum, R. C., Atkinson, C., Malarkey, W. B. & Glaser, R. (2003) Proc. Natl. Acad. Sci. USA 100, 9090-9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, S., Tyrell, D. A. & Smith, A. P. (1991) N. Engl. J. Med. 325, 606-612. [DOI] [PubMed] [Google Scholar]
- 10.Mills, P. J., Dimsdale, J. E., Nelesen, R. A. & Dillon, E. (1996) J. Psychosom. Res. 40, 417-423. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, S., Tyrell, D. A. & Smith, A. P. (1993) J. Pers. Soc. Psychol. 64, 131-140. [DOI] [PubMed] [Google Scholar]
- 12.Tomarken, A. J., Davidson, R. J., Wheeler, R. E. & Doss, R. C. (1992) J. Pers. Soc. Psychol. 62, 676-687. [DOI] [PubMed] [Google Scholar]
- 13.Tomarken, A. J., Davidson, R. J. & Henriques, J. B. (1990) J. Pers. Soc. Psychol. 59, 791-801. [DOI] [PubMed] [Google Scholar]
- 14.Davidson, R. J. & Fox, N. A. (1989) J. Abnorm. Psychol. 98, 127-131. [DOI] [PubMed] [Google Scholar]
- 15.Kang, D. H., Davidson, R. J., Coe, C. C., Wheeler, R. E., Tomarken, A. J. & Ershler, W. B. (1991) Behav. Neurosci. 105, 860-869. [DOI] [PubMed] [Google Scholar]
- 16.Davidson, R. J., Coe, C. C., Dolski, I. & Donzella, B. (1999) Brain Behav. Immun. 13, 93-108. [DOI] [PubMed] [Google Scholar]
- 17.Henriques, J. B. & Davidson, R. J. (1991) J. Abnorm. Psychol. 100, 535-545. [DOI] [PubMed] [Google Scholar]
- 18.Henriques, J. B. & Davidson, R. J. (1990) J. Abnorm. Psychol. 99, 22-31. [DOI] [PubMed] [Google Scholar]
- 19.Irwin, M. (2002) Brain Behav. Immun. 16, 1-16. [DOI] [PubMed] [Google Scholar]
- 20.Miller, A. H. (1998) Psychiatr. Clin. North Am. 21, 443-463. [DOI] [PubMed] [Google Scholar]
- 21.Musselman, D. L., Evans, D. L. & Nemeroff, C. B. (1998) Arch. Gen. Psychiatry 55, 580-592. [DOI] [PubMed] [Google Scholar]
- 22.Dickens, C., McGowan, L., Clark-Carter, D. & Creed, F. (2002) Psychosom. Med. 64, 52-60. [DOI] [PubMed] [Google Scholar]
- 23.Maes, M., Bosmans, E., De Jongh, R., Kenis, G., Vandoolaeghe, E. & Neels, H. (1995) Cytokine 9, 853-858. [DOI] [PubMed] [Google Scholar]
- 24.Ferrucci, L., Harris, T. B., Guralnik, J. M., Tracy R. P., Corti M. C., Cohen, H. J., Penninx, B., Pahor, M., Wallace, R. & Havlik, R. J. (1999) J. Am. Geriatr. Soc. 47, 639-646. [DOI] [PubMed] [Google Scholar]
- 25.Weaver, J. D., Huang, M. H., Albert, M., Harris, T., Rowe, J. W. & Seeman, T. E. (2002) Neurology 59, 371-378. [DOI] [PubMed] [Google Scholar]
- 26.Irwin, M., Costlow, C., Williams, H., Artin, K. H., Chan, C. Y., Stinson, D. L., Levin, M. J., Hayward, A. R. & Oxman, M. N. (1998) J. Infect. Dis. 178, S104-S108. [DOI] [PubMed] [Google Scholar]
- 27.Marsland, A. L., Cohen, S., Rabin, B. S. & Manuck, S. B. (2001) Health Psychol. 20, 4-11. [PubMed] [Google Scholar]
- 28.Chapman, L. J. & Chapman, J. P. (1987) Brain Cognit. 6, 175-183. [DOI] [PubMed] [Google Scholar]
- 29.Beaton, A. A. (2003) in The Asymmetrical Brain, eds. Hugdahl, K. & Davidson, R. J. (MIT Press, Cambridge, MA), pp. 105-158.
- 30.Tomarken, A. J., Davidson, R. J., Wheeler, R. E. & Kinney, L. (1992) Psychophysiology 29, 576-592. [DOI] [PubMed] [Google Scholar]
- 31.Davidson, R. J., Jackson, D. C., Larson, C. L. (2000) in Principles of Psychophysiology, eds. Cacioppo, J. T., Bernston, G. G. & Tassinary, L. G. (Cambridge Univ. Press, New York), pp. 27-52.
- 32.Jackson, D. C., Malmstadt, J. R., Larson, C. L. & Davidson, R. J. (2000) Psychophysiology 37, 515-522. [PubMed] [Google Scholar]
- 33.Kendal, A. P., Pereira, M. S. & Skehel, J. J. (1982) Concepts and Procedures for Laboratory-Based Influenza Surveillance (Centers for Disease Control, U.S. Dept. of Health and Human Services, Atlanta), pp. 17-35.
- 34.Zalcman, S., Green-Johnson, J. M., Murray, L., Nance, D. M., Dyck, D., Anisman, H. & Greenberg, A. H. (1994) Brain Res. 643, 40-49. [DOI] [PubMed] [Google Scholar]
- 35.Merali, Z., Lacosta, S. & Anisman, H. (1997) Brain Res. 761, 225-235. [DOI] [PubMed] [Google Scholar]
- 36.Berridge, C. W. (2003) in The Asymmetrical Brain, eds. Hugdahl, K. & Davidson, R. J. (MIT Press, Cambridge, MA), pp. 69-103.
- 37.Renoux, G. & Biziere, K. (1991) in Psychoneuroimmunology, eds. Ader, R., Felten, D. & Cohen, N. (Academic, San Diego), 2nd ed., pp. 127-141.
- 38.Vlajković, S., Nikolić, V., Nikolić, A., Snežana, M. & Janković, B. D. (1994) Int. J. Neurosci. 78, 123-134. [DOI] [PubMed] [Google Scholar]
- 39.Roszman, T. L., Jackson, J. C., Cross, R. J., Titus, M. J., Markesbery, W. R. & Brooks, W. T. (1985) J. Immunol. 135, 769s-772s. [PubMed] [Google Scholar]
- 40.Nisticó, G., Caroleo, M. C., Artitrio, M. & Pulvirenti, L. (1994) Neuroimmunomodulation 1, 174-180. [DOI] [PubMed] [Google Scholar]
- 41.Kalin, N. H., Larson, C., Shelton, S. E. & Davidson, R. J. (1998) Behav. Neurosci. 112, 286-292. [DOI] [PubMed] [Google Scholar]
- 42.Buss, K. A., Malmstadt, J. R., Dolski, I., Kalin, N. H., Goldsmith, H. H. & Davidson, R. J. (2003) Behav. Neurosci. 117, 11-20. [DOI] [PubMed] [Google Scholar]
- 43.Kalin, N. H., Shelton, S. E. & Davidson, R. J. (2000) Biol. Psychiatry 47, 579-585. [DOI] [PubMed] [Google Scholar]
- 44.Jackson, D. C., Mueller, C. J., Dolski, I., Dalton, K. M., Nitschke, J. B., Urry, H. L., Rosenkranz, M. A., Ryff, C. D., Singer, B. H. & Davidson, R. J. (2003) Psychol. Sci., in press. [DOI] [PubMed]
- 45.Walsh, V. & Cowey, A. (2000) Nat. Rev. Neurosci. 1, 73-79. [DOI] [PubMed] [Google Scholar]