Abstract
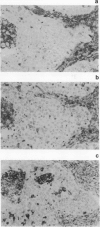
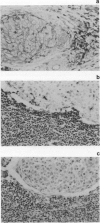
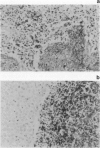
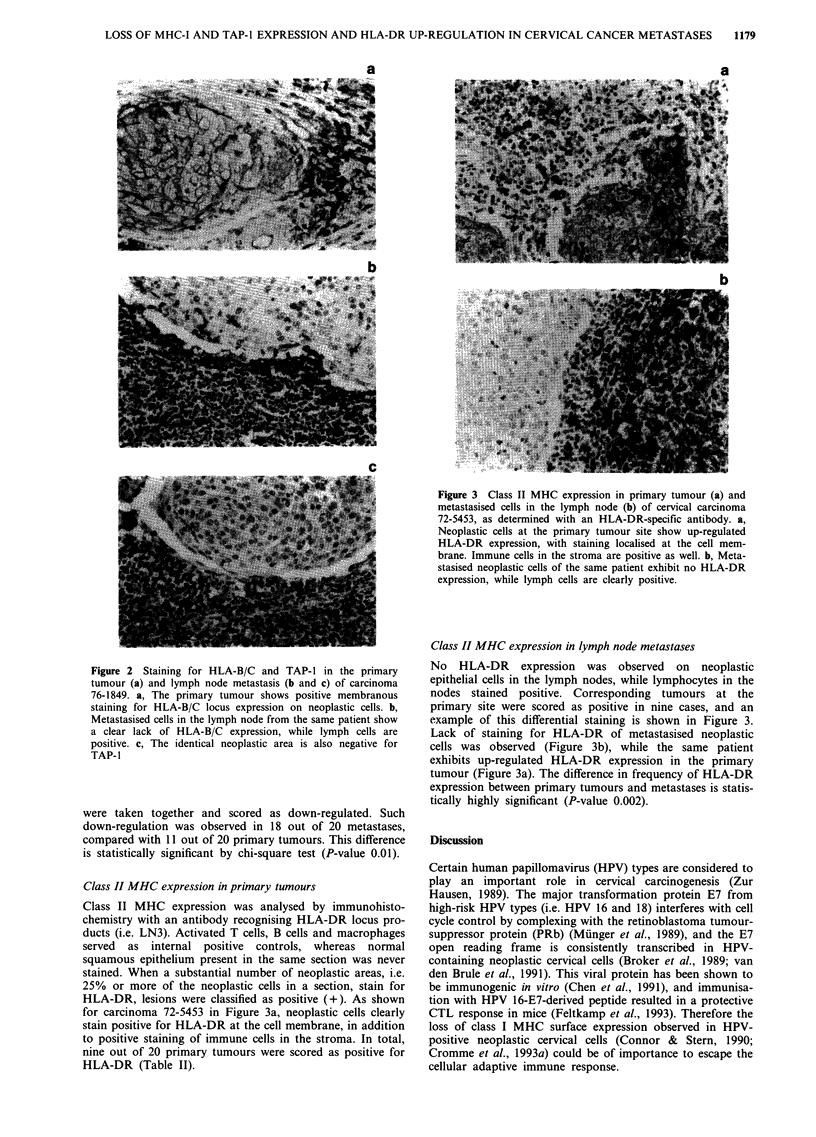
In previous studies we have shown down-regulation of class I major histocompatibility complex (MHC) expression in a significant proportion of primary cervical carcinomas, which was found to be strongly correlated with loss of expression of the transporter associated with antigen presentation (TAP). By contrast, class II MHC expression was frequently up-regulated on neoplastic keratinocytes in these malignancies. In order to investigate whether these changes are associated with biological behaviour of the tumours, 20 cervical carcinomas were analyzed for MHC (HLA-A, HLA-B/C, HLA-DR) and TAP-1 expression in the primary tumours and in lymph node metastases by immunohistochemistry. The results showed a significant increase in the prevalence of HLA-A and HLA-B/C down-regulation in metastasised neoplastic cells as compared with the primary tumour (P = 0.01). In all cases this was accompanied by loss of TAP-1 expression. Up-regulated HLA-DR expression was found exclusively in primary tumours and was absent in the corresponding metastases (P = 0.002). These data are consistent with the hypothesis that loss of TAP-1 and the consequent down-regulation of class I MHC expression provides a selective advantage for neoplastic cervical cells during metastasis. Furthermore, the lack of class II MHC expression in metastasised cells either reflects a different local lymphokine production or indicates that these cells may have escaped CD4+ cytotoxic T-lymphocyte (CTL)-mediated killing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Algarra I., Gaforio J. J., Garrido A., Mialdea M. J., Pérez M., Garrido F. Heterogeneity of MHC-class-I antigens in clones of methylcholanthrene-induced tumors. Implications for local growth and metastasis. Int J Cancer Suppl. 1991;6:73–81. doi: 10.1002/ijc.2910470716. [DOI] [PubMed] [Google Scholar]
- Altmann A., Jochmus-Kudielka I., Frank R., Gausepohl H., Moebius U., Gissmann L., Meuer S. C. Definition of immunogenic determinants of the human papillomavirus type 16 nucleoprotein E7. Eur J Cancer. 1992;28(2-3):326–333. doi: 10.1016/s0959-8049(05)80047-4. [DOI] [PubMed] [Google Scholar]
- Bal V., McIndoe A., Denton G., Hudson D., Lombardi G., Lamb J., Lechler R. Antigen presentation by keratinocytes induces tolerance in human T cells. Eur J Immunol. 1990 Sep;20(9):1893–1897. doi: 10.1002/eji.1830200904. [DOI] [PubMed] [Google Scholar]
- Chen L. P., Thomas E. K., Hu S. L., Hellström I., Hellström K. E. Human papillomavirus type 16 nucleoprotein E7 is a tumor rejection antigen. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):110–114. doi: 10.1073/pnas.88.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha A., Cabrera T., Ruiz-Cabello F., Garrido F. Can the HLA phenotype be used as a prognostic factor in breast carcinomas? Int J Cancer Suppl. 1991;6:146–154. doi: 10.1002/ijc.2910470726. [DOI] [PubMed] [Google Scholar]
- Connor M. E., Stern P. L. Loss of MHC class-I expression in cervical carcinomas. Int J Cancer. 1990 Dec 15;46(6):1029–1034. doi: 10.1002/ijc.2910460614. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Fuks Z., Drobnjak M., Moreno C., Eisenbach L., Feldman M. Expression of HLA-A,B,C antigens on primary and metastatic tumor cell populations of human carcinomas. Cancer Res. 1991 Dec 1;51(23 Pt 1):6372–6380. [PubMed] [Google Scholar]
- Cromme F. V., Meijer C. J., Snijders P. J., Uyterlinde A., Kenemans P., Helmerhorst T., Stern P. L., van den Brule A. J., Walboomers J. M. Analysis of MHC class I and II expression in relation to presence of HPV genotypes in premalignant and malignant cervical lesions. Br J Cancer. 1993 Jun;67(6):1372–1380. doi: 10.1038/bjc.1993.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromme F. V., Snijders P. J., van den Brule A. J., Kenemans P., Meijer C. J., Walboomers J. M. MHC class I expression in HPV 16 positive cervical carcinomas is post-transcriptionally controlled and independent from c-myc overexpression. Oncogene. 1993 Nov;8(11):2969–2975. [PubMed] [Google Scholar]
- De Baetselier P., Katzav S., Gorelik E., Feldman M., Segal S. Differential expression of H-2 gene products in tumour cells in associated with their metastatogenic properties. Nature. 1980 Nov 13;288(5787):179–181. doi: 10.1038/288179a0. [DOI] [PubMed] [Google Scholar]
- Esteban F., Concha A., Huelin C., Pérez-Ayala M., Pedrinaci S., Ruiz-Cabello F., Garrido F. Histocompatibility antigens in primary and metastatic squamous cell carcinoma of the larynx. Int J Cancer. 1989 Mar 15;43(3):436–442. doi: 10.1002/ijc.2910430316. [DOI] [PubMed] [Google Scholar]
- Esteban F., Ruiz-Cabello F., Concha A., Pérez-Ayala M., Sánchez-Rozas J. A., Garrido F. HLA-DR expression is associated with excellent prognosis in squamous cell carcinoma of the larynx. Clin Exp Metastasis. 1990 Jul-Aug;8(4):319–328. doi: 10.1007/BF01810678. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Rammensee H. G. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature. 1990 Nov 15;348(6298):248–251. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- Gaspari A. A., Jenkins M. K., Katz S. I. Class II MHC-bearing keratinocytes induce antigen-specific unresponsiveness in hapten-specific Th1 clones. J Immunol. 1988 Oct 1;141(7):2216–2220. [PubMed] [Google Scholar]
- Glew S. S., Connor M. E., Snijders P. J., Stanbridge C. M., Buckley C. H., Walboomers J. M., Meijer C. J., Stern P. L. HLA expression in pre-invasive cervical neoplasia in relation to human papilloma virus infection. Eur J Cancer. 1993;29A(14):1963–1970. doi: 10.1016/0959-8049(93)90453-m. [DOI] [PubMed] [Google Scholar]
- Glew S. S., Duggan-Keen M., Cabrera T., Stern P. L. HLA class II antigen expression in human papillomavirus-associated cervical cancer. Cancer Res. 1992 Jul 15;52(14):4009–4016. [PubMed] [Google Scholar]
- Goldberg A. L., Rock K. L. Proteolysis, proteasomes and antigen presentation. Nature. 1992 Jun 4;357(6377):375–379. doi: 10.1038/357375a0. [DOI] [PubMed] [Google Scholar]
- Jaraquemada D., Marti M., Long E. O. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II-restricted T cells. J Exp Med. 1990 Sep 1;172(3):947–954. doi: 10.1084/jem.172.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzav S., Segal S., Feldman M. Immuno-selection in vivo of H-2D phenotypic variants from a metastatic clone of sarcoma cells results in cell lines of altered metastatic competence. Int J Cancer. 1984 Mar 15;33(3):407–415. doi: 10.1002/ijc.2910330320. [DOI] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Lopez Nevot M. A., Garcia E., Pareja E., Bonal F. J., Martin J., Ruiz-Cabello F., Serrano S., Garrido F. Differential expression of HLA class I and II antigens in primary and metastatic melanomas. J Immunogenet. 1986 Apr-Jun;13(2-3):219–227. doi: 10.1111/j.1744-313x.1986.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Maiman M., Fruchter R. G., Guy L., Cuthill S., Levine P., Serur E. Human immunodeficiency virus infection and invasive cervical carcinoma. Cancer. 1993 Jan 15;71(2):402–406. doi: 10.1002/1097-0142(19930115)71:2<402::aid-cncr2820710222>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J. J., Momburg F., Hämmerling G. J. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science. 1993 Aug 6;261(5122):769–771. doi: 10.1126/science.8342042. [DOI] [PubMed] [Google Scholar]
- Paterson A. C., Sciot R., Kew M. C., Callea F., Dusheiko G. M., Desmet V. J. HLA expression in human hepatocellular carcinoma. Br J Cancer. 1988 Apr;57(4):369–373. doi: 10.1038/bjc.1988.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo N. P., Esquivel F., Kawakami Y., Yewdell J. W., Mulé J. J., Rosenberg S. A., Bennink J. R. Identification of human cancers deficient in antigen processing. J Exp Med. 1993 Feb 1;177(2):265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiter D. J., Bröcker E. B., Ferrone S. Expression and susceptibility to modulation by interferons of HLA class I and II antigens on melanoma cells. Immunohistochemical analysis and clinical relevance. J Immunogenet. 1986 Apr-Jun;13(2-3):229–234. doi: 10.1111/j.1744-313x.1986.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Cabello F., Klein E., Garrido F. MHC antigens on human tumors. Immunol Lett. 1991 Aug;29(3):181–189. doi: 10.1016/0165-2478(91)90168-a. [DOI] [PubMed] [Google Scholar]
- Sakai K., Takiguchi M., Mori S., Kobori O., Morioka Y., Inoko H., Sekiguchi M., Kano K. Expression and function of class II antigens on gastric carcinoma cells and gastric epithelia: differential expression of DR, DQ, and DP antigens. J Natl Cancer Inst. 1987 Nov;79(5):923–932. [PubMed] [Google Scholar]
- Shepherd J. C., Schumacher T. N., Ashton-Rickardt P. G., Imaeda S., Ploegh H. L., Janeway C. A., Jr, Tonegawa S. TAP1-dependent peptide translocation in vitro is ATP dependent and peptide selective. Cell. 1993 Aug 13;74(3):577–584. doi: 10.1016/0092-8674(93)80058-m. [DOI] [PubMed] [Google Scholar]
- Sillman F. H., Sedlis A. Anogenital papillomavirus infection and neoplasia in immunodeficient women. Obstet Gynecol Clin North Am. 1987 Jun;14(2):537–558. [PubMed] [Google Scholar]
- Snijders P. J., van den Brule A. J., Schrijnemakers H. F., Snow G., Meijer C. J., Walboomers J. M. The use of general primers in the polymerase chain reaction permits the detection of a broad spectrum of human papillomavirus genotypes. J Gen Virol. 1990 Jan;71(Pt 1):173–181. doi: 10.1099/0022-1317-71-1-173. [DOI] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Bahram S., Arnold D., Blanck G., Mellins E., Pious D., DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990 Dec 20;348(6303):744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- Stam N. J., Spits H., Ploegh H. L. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986 Oct 1;137(7):2299–2306. [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Hanson I., Mockridge I., Beck S., Townsend A., Kelly A. Sequences encoded in the class II region of the MHC related to the 'ABC' superfamily of transporters. Nature. 1990 Dec 20;348(6303):741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- Zöller M., Strubel A., Hämmerling G., Andrighetto G., Raz A., Ben-Ze'ev A. Interferon-gamma treatment of B16 melanoma cells: opposing effects for non-adaptive and adaptive immune defense and its reflection by metastatic spread. Int J Cancer. 1988 Feb 15;41(2):256–266. doi: 10.1002/ijc.2910410217. [DOI] [PubMed] [Google Scholar]
- van den Brule A. J., Cromme F. V., Snijders P. J., Smit L., Oudejans C. B., Baak J. P., Meijer C. J., Walboomers J. M. Nonradioactive RNA in situ hybridization detection of human papillomavirus 16-E7 transcripts in squamous cell carcinomas of the uterine cervix using confocal laser scan microscopy. Am J Pathol. 1991 Nov;139(5):1037–1045. [PMC free article] [PubMed] [Google Scholar]
- van den Brule A. J., Meijer C. J., Bakels V., Kenemans P., Walboomers J. M. Rapid detection of human papillomavirus in cervical scrapes by combined general primer-mediated and type-specific polymerase chain reaction. J Clin Microbiol. 1990 Dec;28(12):2739–2743. doi: 10.1128/jcm.28.12.2739-2743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ingh H. F., Ruiter D. J., Griffioen G., van Muijen G. N., Ferrone S. HLA antigens in colorectal tumours--low expression of HLA class I antigens in mucinous colorectal carcinomas. Br J Cancer. 1987 Feb;55(2):125–130. doi: 10.1038/bjc.1987.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989 Sep 1;49(17):4677–4681. [PubMed] [Google Scholar]