Abstract
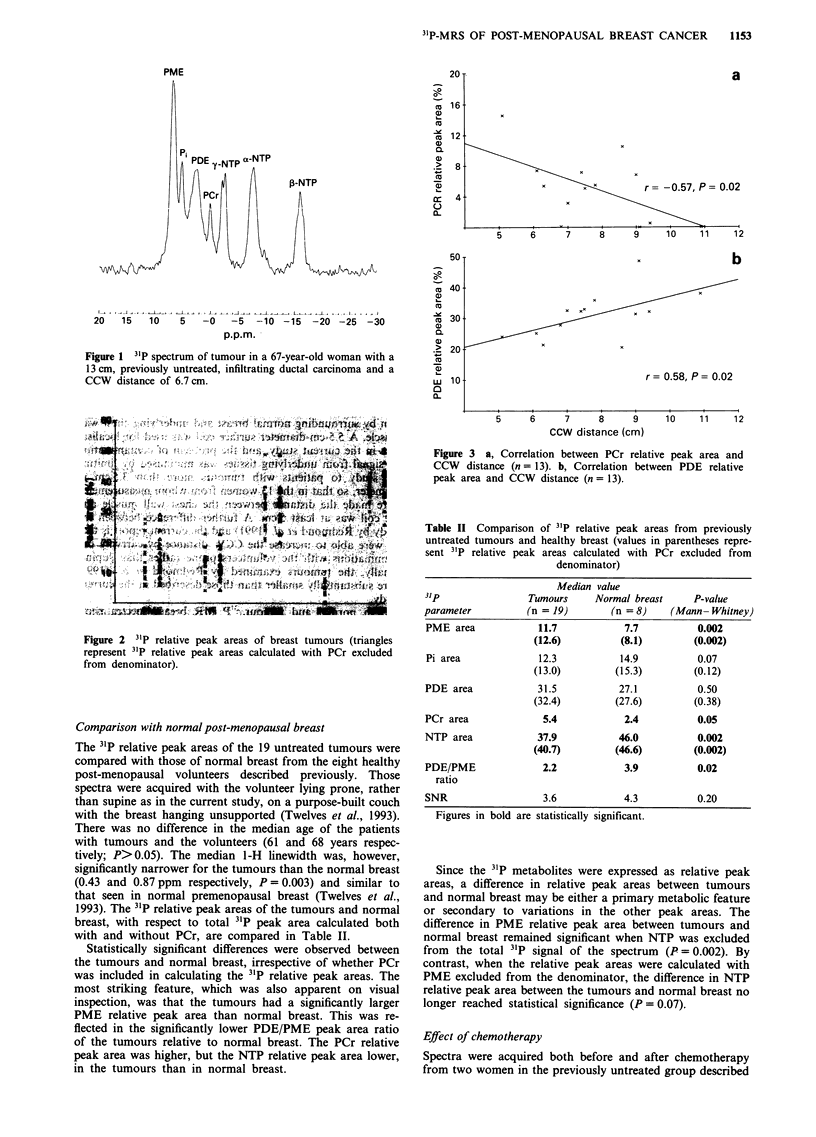
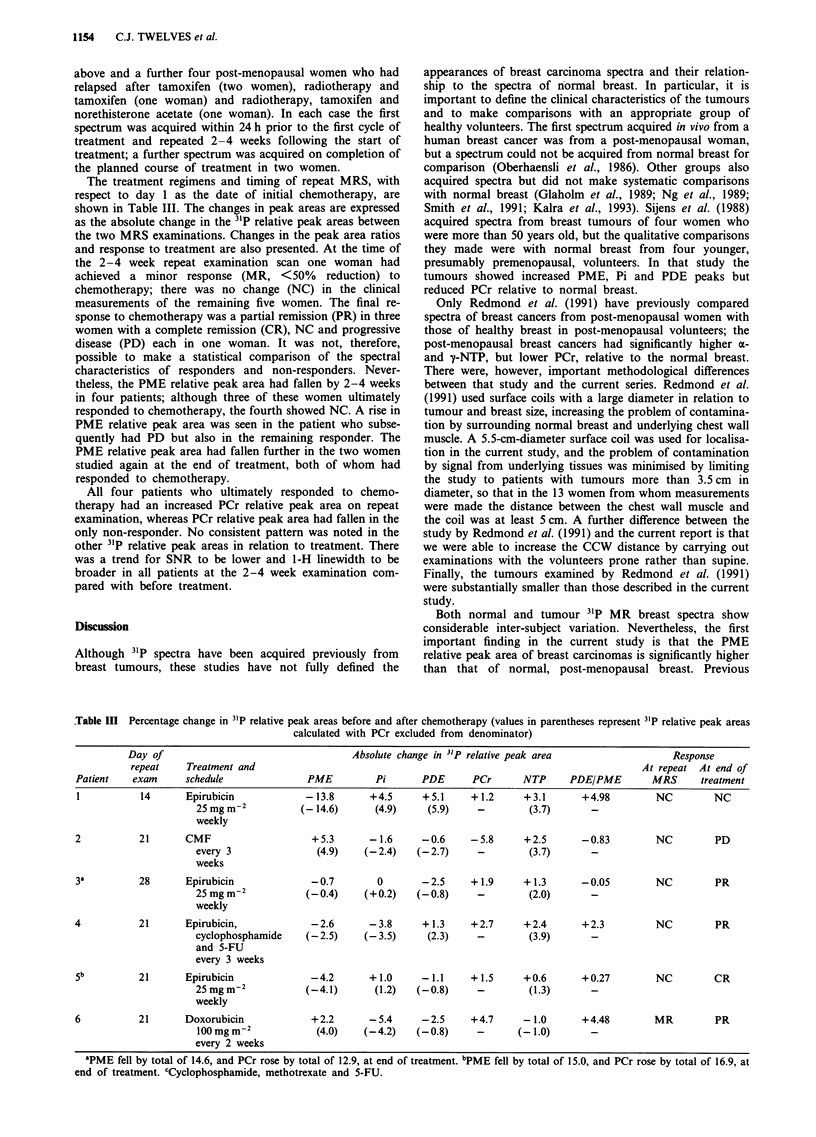
We have studied the metabolism of 31P-containing metabolites of post-menopausal breast cancers in vivo using magnetic resonance spectroscopy (MRS) and a 5.5 cm surface coil. Spectra were acquired from 23 diameter. The spectra of the 19 previously untreated tumours had significantly higher phosphomonoester (PME) 31P relative peak areas than the normal breasts of eight post-menopausal women (11.7% and 7.7% respectively, P = 0.002). Although an increased PME relative peak area was characteristic of malignancy, PME relative peak area is similarly raised in lactating breast and, therefore, not a specific feature of cancer. An apparently lower nucleotide triphosphate (NTP) relative peak area in tumours than healthy postmenopausal breast was secondary to the differences in PME relative peak area; contamination by signal from chest wall muscle probably accounts for the ostensibly higher phosphocreatine (PCr) relative peak area of the tumours. Spectroscopy was repeated following chemotherapy in six women. An increase in PCr relative peak area was seen in all five patients who responded, but again this may represent increased contamination secondary to changes in tumour size. A fall in PME relative peak area was noted in four responders, but also one non-responder, so this finding may not be sufficiently specific to be of use clinically. Further studies are need to elucidate fully the role of MRS in breast cancer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman J. J., Grove T. H., Wong G. G., Gadian D. G., Radda G. K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980 Jan 10;283(5743):167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. S., Lyon R. C., Chen C., Faustino P. J., Batist G., Shoemaker M., Rubalcaba E., Cowan K. H. Differences in phosphate metabolite levels in drug-sensitive and -resistant human breast cancer cell lines determined by 31P magnetic resonance spectroscopy. Cancer Res. 1986 Aug;46(8):4087–4090. [PubMed] [Google Scholar]
- Degani H., Horowitz A., Itzchak Y. Breast tumors: evaluation with P-31 MR spectroscopy. Radiology. 1986 Oct;161(1):53–55. doi: 10.1148/radiology.161.1.3020609. [DOI] [PubMed] [Google Scholar]
- Evanochko W. T., Ng T. C., Lilly M. B., Lawson A. J., Corbett T. H., Durant J. R., Glickson J. D. In vivo 31P NMR study of the metabolism of murine mammary 16/C adenocarcinoma and its response to chemotherapy, x-radiation, and hyperthermia. Proc Natl Acad Sci U S A. 1983 Jan;80(2):334–338. doi: 10.1073/pnas.80.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evelhoch J. L., Keller N. A., Corbett T. H. Response-specific adriamycin sensitivity markers provided by in vivo 31P nuclear magnetic resonance spectroscopy in murine mammary adenocarcinomas. Cancer Res. 1987 Jul 1;47(13):3396–3401. [PubMed] [Google Scholar]
- Glaholm J., Leach M. O., Collins D. J., Mansi J., Sharp J. C., Madden A., Smith I. E., McCready V. R. In-vivo 31P magnetic resonance spectroscopy for monitoring treatment response in breast cancer. Lancet. 1989 Jun 10;1(8650):1326–1327. doi: 10.1016/s0140-6736(89)92717-7. [DOI] [PubMed] [Google Scholar]
- Hoult D. I., Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974 Nov 22;252(5481):285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- Kalra R., Wade K. E., Hands L., Styles P., Camplejohn R., Greenall M., Adams G. E., Harris A. L., Radda G. K. Phosphomonoester is associated with proliferation in human breast cancer: a 31P MRS study. Br J Cancer. 1993 May;67(5):1145–1153. doi: 10.1038/bjc.1993.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenkinski R. E., Allman T., Scheiner J. D., Deming S. N. An automated iterative algorithm for the quantitative analysis of in vivo spectra based on the simplex optimization method. Magn Reson Med. 1989 Jun;10(3):338–348. doi: 10.1002/mrm.1910100306. [DOI] [PubMed] [Google Scholar]
- Lowry M., Porter D. A., Twelves C. J., Heasley P. E., Smith M. A., Richards M. A. Visibility of phospholipids in 31P NMR spectra of human breast tumours in vivo. NMR Biomed. 1992 Jan-Feb;5(1):37–42. doi: 10.1002/nbm.1940050107. [DOI] [PubMed] [Google Scholar]
- Merchant T. E., Gierke L. W., Meneses P., Glonek T. 31P magnetic resonance spectroscopic profiles of neoplastic human breast tissues. Cancer Res. 1988 Sep 15;48(18):5112–5118. [PubMed] [Google Scholar]
- Merchant T. E., Meneses P., Gierke L. W., Den Otter W., Glonek T. 31P magnetic resonance phospholipid profiles of neoplastic human breast tissues. Br J Cancer. 1991 May;63(5):693–698. doi: 10.1038/bjc.1991.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. C., Grundfest S., Vijayakumar S., Baldwin N. J., Majors A. W., Karalis I., Meaney T. F., Shin K. H., Thomas F. J., Tubbs R. Therapeutic response of breast carcinoma monitored by 31P MRS in situ. Magn Reson Med. 1989 Apr;10(1):125–134. doi: 10.1002/mrm.1910100112. [DOI] [PubMed] [Google Scholar]
- Oberhaensli R. D., Hilton-Jones D., Bore P. J., Hands L. J., Rampling R. P., Radda G. K. Biochemical investigation of human tumours in vivo with phosphorus-31 magnetic resonance spectroscopy. Lancet. 1986 Jul 5;2(8497):8–11. doi: 10.1016/s0140-6736(86)92558-4. [DOI] [PubMed] [Google Scholar]
- Redmond O. M., Stack J. P., O'Connor N. G., Carney D. N., Dervan P. A., Hurson B. J., Ennis J. T. 31P MRS as an early prognostic indicator of patient response to chemotherapy. Magn Reson Med. 1992 May;25(1):30–44. doi: 10.1002/mrm.1910250104. [DOI] [PubMed] [Google Scholar]
- Redmond O. M., Stack J. P., O'Connor N. G., Codd M. B., Ennis J. T. In vivo phosphorus-31 magnetic resonance spectroscopy of normal and pathological breast tissues. Br J Radiol. 1991 Mar;64(759):210–216. doi: 10.1259/0007-1285-64-759-210. [DOI] [PubMed] [Google Scholar]
- Ruiz-Cabello J., Cohen J. S. Phospholipid metabolites as indicators of cancer cell function. NMR Biomed. 1992 Sep-Oct;5(5):226–233. doi: 10.1002/nbm.1940050506. [DOI] [PubMed] [Google Scholar]
- Sharp J. C., Leach M. O. Conformal NMR spectroscopy: accurate localization to noncuboidal volumes with optimum SNR. Magn Reson Med. 1989 Sep;11(3):376–388. doi: 10.1002/mrm.1910110312. [DOI] [PubMed] [Google Scholar]
- Sijens P. E., Wijrdeman H. K., Moerland M. A., Bakker C. J., Vermeulen J. W., Luyten P. R. Human breast cancer in vivo: H-1 and P-31 MR spectroscopy at 1.5 T. Radiology. 1988 Dec;169(3):615–620. doi: 10.1148/radiology.169.3.2847230. [DOI] [PubMed] [Google Scholar]
- Smith T. A., Glaholm J., Leach M. O., Machin L., Collins D. J., Payne G. S., McCready V. R. A comparison of in vivo and in vitro 31P NMR spectra from human breast tumours: variations in phospholipid metabolism. Br J Cancer. 1991 Apr;63(4):514–516. doi: 10.1038/bjc.1991.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twelves C. J., Lowry M., Porter D. A., Dobbs N. A., Graves P. E., Smith M. A., Richards M. A. Phosphorus-31 metabolism of human breast--an in vivo magnetic resonance spectroscopic study at 1.5 Tesla. Br J Radiol. 1994 Jan;67(793):36–45. doi: 10.1259/0007-1285-67-793-36. [DOI] [PubMed] [Google Scholar]


