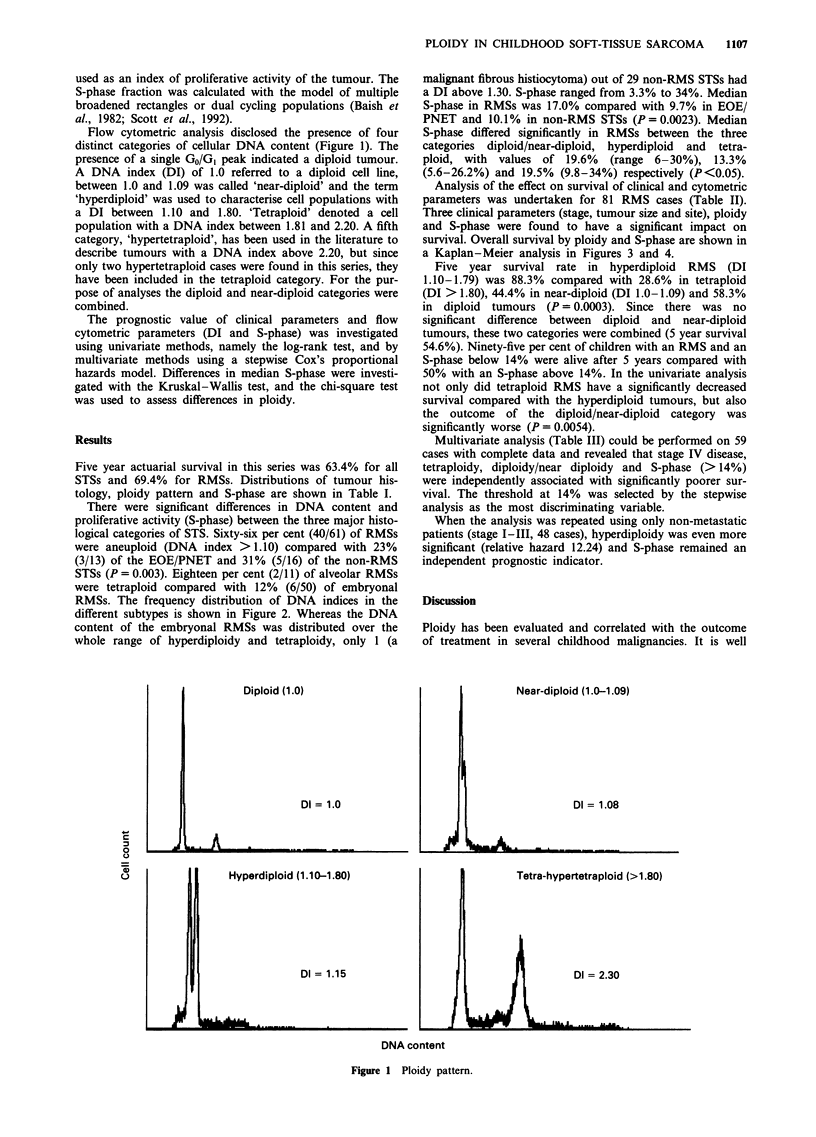
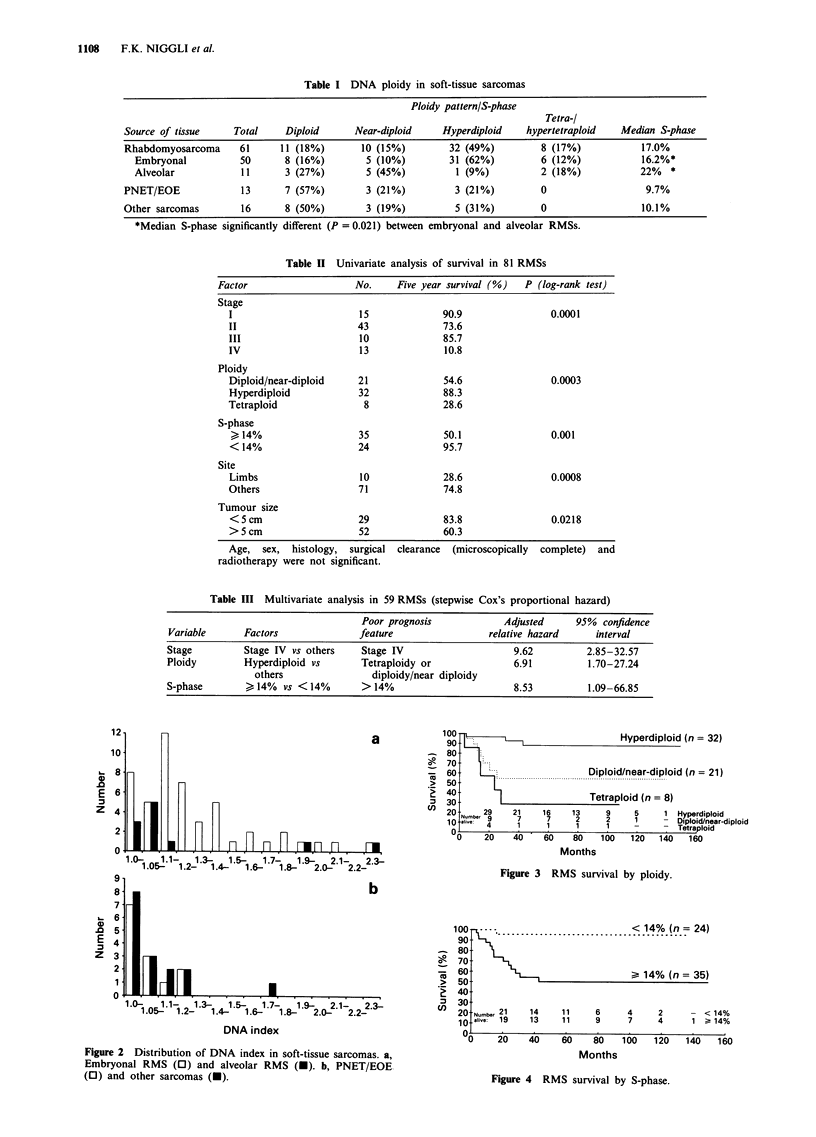
Abstract
The value of DNA ploidy as a prognostic indicator is well established in many cancers, but recent studies in childhood rhabdomyosarcoma (RMS) have been contradictory. In a retrospective study of 128 cases of soft-tissue sarcoma (STS) diagnosed since 1980, the prognostic value of clinical, histological and flow cytometric parameters was compared, using univariate and multivariate methods. Eighty-one RMSs, 18 extraosseous Ewing's (EOE)/peripheral neuroectodermal tumours (PNETs) and 29 other non-RMS STSs were histologically and clinically reviewed. Five year actuarial survival was 63.4% for all STSs and 69.4% for RMSs. Paraffin-embedded tissue blocks were available for flow cytometry in 90 cases. Of the RMSs, 65.5% were aneuploid [DNA index (DI) > 1.1] compared with 23% of the EOE/PNETs and 31% of non-RMS STSs. Median S-phase was also significantly higher in RMSs (17.0%) than in other STSs (10.8%) (P = 0.0023). Univariate analysis in RMSs showed that stage, ploidy status, S-phase, site and tumour size all had a significant impact on survival. In multivariate analysis of 59 cases of RMS, one clinical and two flow cytometric parameters were independently associated with poor prognosis. These were stage (IV), nonhyperdiploidy (DI < 1.10 and > 1.8) and a high rate of proliferative activity (S-phase > 14.0%). These results confirm that ploidy and S-phase are important new prognostic indicators in rhabdomyosarcoma.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baisch H., Beck H. P., Christensen I. J., Hartmann N. R., Fried J., Dean P. N., Gray J. W., Jett J. H., Johnston D. A., White R. A. A comparison of mathematical methods for the analysis of DNA histograms obtained by flow cytometry. Cell Tissue Kinet. 1982 May;15(3):235–249. doi: 10.1111/j.1365-2184.1982.tb01043.x. [DOI] [PubMed] [Google Scholar]
- Barrantes J. C., Muir K. R., Toyn C. E., Parkes S. E., Cameron A. H., Marsden H. B., Raafat F., Mann J. R. Thirty-year population-based review of childhood renal tumours with an assessment of prognostic features including tumour DNA characteristics. Med Pediatr Oncol. 1993;21(1):24–30. doi: 10.1002/mpo.2950210106. [DOI] [PubMed] [Google Scholar]
- Boyle E. T., Jr, Reiman H. M., Kramer S. A., Kelalis P. P., Rainwater L. M., Lieber M. M. Embryonal rhabdomyosarcoma of bladder and prostate: nuclear DNA patterns studied by flow cytometry. J Urol. 1988 Nov;140(5 Pt 2):1119–1121. doi: 10.1016/s0022-5347(17)41976-8. [DOI] [PubMed] [Google Scholar]
- Dias P., Kumar P., Marsden H. B., Gattamaneni H. R., Kumar S. Prognostic relevance of DNA ploidy in rhabdomyosarcomas and other sarcomas of childhood. Anticancer Res. 1992 Jul-Aug;12(4):1173–1177. [PubMed] [Google Scholar]
- Douglass E. C., Look A. T., Webber B., Parham D., Wilimas J. A., Green A. A., Roberson P. K. Hyperdiploidy and chromosomal rearrangements define the anaplastic variant of Wilms' tumor. J Clin Oncol. 1986 Jun;4(6):975–981. doi: 10.1200/JCO.1986.4.6.975. [DOI] [PubMed] [Google Scholar]
- Frierson H. F., Jr Flow cytometric analysis of ploidy in solid neoplasms: comparison of fresh tissues with formalin-fixed paraffin-embedded specimens. Hum Pathol. 1988 Mar;19(3):290–294. doi: 10.1016/s0046-8177(88)80521-5. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Herman C. J. Cytometric DNA analysis in the management of cancer. Clinical and laboratory considerations. Cancer. 1992 Mar 15;69(6 Suppl):1553–1556. doi: 10.1002/1097-0142(19920315)69:6+<1553::aid-cncr2820691308>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Huddart S. N., Muir K. R., Parkes S. E., Mann J. R., Stevens M. C., Raafat F., Smith K. Retrospective study of prognostic value of DNA ploidy and proliferative activity in neuroblastoma. J Clin Pathol. 1993 Dec;46(12):1101–1104. doi: 10.1136/jcp.46.12.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioniemi O. P. Comparison of fresh and paraffin-embedded tissue as starting material for DNA flow cytometry and evaluation of intratumor heterogeneity. Cytometry. 1988 Mar;9(2):164–169. doi: 10.1002/cyto.990090211. [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Kanda N., Maseki N., Sakurai M., Tsuchida Y., Takeda T., Okabe I., Sakurai M. Different karyotypic patterns in early and advanced stage neuroblastomas. Cancer Res. 1987 Jan 1;47(1):311–318. [PubMed] [Google Scholar]
- Kowal-Vern A., Gonzalez-Crussi F., Turner J., Trujillo Y. P., Chou P., Herman C., Castelli M., Walloch J. Flow and image cytometric DNA analysis in rhabdomyosarcoma. Cancer Res. 1990 Sep 15;50(18):6023–6027. [PubMed] [Google Scholar]
- Look A. T., Hayes F. A., Nitschke R., McWilliams N. B., Green A. A. Cellular DNA content as a predictor of response to chemotherapy in infants with unresectable neuroblastoma. N Engl J Med. 1984 Jul 26;311(4):231–235. doi: 10.1056/NEJM198407263110405. [DOI] [PubMed] [Google Scholar]
- Look A. T., Roberson P. K., Williams D. L., Rivera G., Bowman W. P., Pui C. H., Ochs J., Abromowitch M., Kalwinsky D., Dahl G. V. Prognostic importance of blast cell DNA content in childhood acute lymphoblastic leukemia. Blood. 1985 May;65(5):1079–1086. [PubMed] [Google Scholar]
- Maurer H. M., Gehan E. A., Beltangady M., Crist W., Dickman P. S., Donaldson S. S., Fryer C., Hammond D., Hays D. M., Herrmann J. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993 Mar 1;71(5):1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Merkel D. E., Dressler L. G., McGuire W. L. Flow cytometry, cellular DNA content, and prognosis in human malignancy. J Clin Oncol. 1987 Oct;5(10):1690–1703. doi: 10.1200/JCO.1987.5.10.1690. [DOI] [PubMed] [Google Scholar]
- Molenaar W. M., Dam-Meiring A., Kamps W. A., Cornelisse C. J. DNA-aneuploidy in rhabdomyosarcomas as compared with other sarcomas of childhood and adolescence. Hum Pathol. 1988 May;19(5):573–579. doi: 10.1016/s0046-8177(88)80207-7. [DOI] [PubMed] [Google Scholar]
- Pappo A. S., Crist W. M., Kuttesch J., Rowe S., Ashmun R. A., Maurer H. M., Newton W. A., Asmar L., Luo X., Shapiro D. N. Tumor-cell DNA content predicts outcome in children and adolescents with clinical group III embryonal rhabdomyosarcoma. The Intergroup Rhabdomyosarcoma Study Committee of the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol. 1993 Oct;11(10):1901–1905. doi: 10.1200/JCO.1993.11.10.1901. [DOI] [PubMed] [Google Scholar]
- Rodary C., Flamant F., Donaldson S. S. An attempt to use a common staging system in rhabdomyosarcoma: a report of an international workshop initiated by the International Society of Pediatric Oncology (SIOP). Med Pediatr Oncol. 1989;17(3):210–215. doi: 10.1002/mpo.2950170308. [DOI] [PubMed] [Google Scholar]
- Rodary C., Gehan E. A., Flamant F., Treuner J., Carli M., Auquier A., Maurer H. Prognostic factors in 951 nonmetastatic rhabdomyosarcoma in children: a report from the International Rhabdomyosarcoma Workshop. Med Pediatr Oncol. 1991;19(2):89–95. doi: 10.1002/mpo.2950190204. [DOI] [PubMed] [Google Scholar]
- Schmidt D., Wiedemann B., Keil W., Sprenger E., Harms D. Flow cytometric analysis of nephroblastomas and related neoplasms. Cancer. 1986 Dec 1;58(11):2494–2500. doi: 10.1002/1097-0142(19861201)58:11<2494::aid-cncr2820581124>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Scott N., Cross D., Plumb M. I., Dixon M. F., Quirke P. An investigation of different methods of cell cycle analysis by flow cytometry in rectal cancer. Br J Cancer. 1992 Jan;65(1):8–10. doi: 10.1038/bjc.1992.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. N., Parham D. M., Douglass E. C., Ashmun R., Webber B. L., Newton W. A., Jr, Hancock M. L., Maurer H. M., Look A. T. Relationship of tumor-cell ploidy to histologic subtype and treatment outcome in children and adolescents with unresectable rhabdomyosarcoma. J Clin Oncol. 1991 Jan;9(1):159–166. doi: 10.1200/JCO.1991.9.1.159. [DOI] [PubMed] [Google Scholar]
- Swanson P. E., Jaszcz W., Nakhleh R. E., Kelly D. R., Dehner L. P. Peripheral primitive neuroectodermal tumors. A flow cytometric analysis with immunohistochemical and ultrastructural observations. Arch Pathol Lab Med. 1992 Nov;116(11):1202–1208. [PubMed] [Google Scholar]
- Yasue M., Tomita T., Engelhard H., Gonzalez-Crussi F., McLone D. G., Bauer K. D. Prognostic importance of DNA ploidy in medulloblastoma of childhood. J Neurosurg. 1989 Mar;70(3):385–391. doi: 10.3171/jns.1989.70.3.0385. [DOI] [PubMed] [Google Scholar]
- Zerbini C., Gelber R. D., Weinberg D., Sallan S. E., Barnes P., Kupsky W., Scott R. M., Tarbell N. J. Prognostic factors in medulloblastoma, including DNA ploidy. J Clin Oncol. 1993 Apr;11(4):616–622. doi: 10.1200/JCO.1993.11.4.616. [DOI] [PubMed] [Google Scholar]


