Abstract
How memory T cells are maintained in vivo is poorly understood. To address this problem, a male-specific peptide (H-Y) was identified and used to activate female anti-H-Y T cells in vitro. Anti-H-Y T cells survived in vivo for at least 70 days in the absence of antigen. This persistence was not because of the intrinsic ability of memory T cells to survive in vivo. Instead, the survival and function of adoptively transferred memory cells was found to require transporter of antigen protein 1-dependent expression of self-peptide/major histocompatibility complex class I molecules in recipient animals. Therefore, it appears that the level of T cell receptor engagement provided by transporter of antigen protein 1-dependent, self-peptide/major histocompatibility complexes is sufficient to maintain the long-term survival and functional phenotype of memory cells in the absence of persistent antigen. These data suggest that positive selection plays a role not only in T cell development but also in the maintenance of T cell memory.
Immunological memory is a characteristic feature of the adaptive immune system and appears to result from the persistence of a heightened reactive state initiated by antigenic challenge. Secondary or memory responses are more rapid in onset and more effective than primary immune responses. The ability to generate a memory response protects organisms from recurrent challenge by pathogens. The means by which T cell memory is maintained is not completely understood. One model postulates that long- term memory is dependent on persistent antigenic stimulation (1, 2). According to this model small amounts of antigen, derived from an initial infection, persist in specialized depots, such as follicular dendritic cells, ensuring that a small subset of T cells is maintained in an activated state long after a pathogen is cleared. However, this view was challenged by the observation that T cells from virally immunized mice, when adoptively transferred to antigen-free animal hosts, gave rise to T cells that responded rapidly to antigen and retained the expression of activation markers (3–5). These results suggest that T cell memory may be because of the presence of long-lived memory cells, which are derived from antigen-activated cells and persist in the absence of antigen.
Mature T cells express T cell receptors (TCRs), which are weakly reactive to self-peptide/major histocompatibility complex (MHC) molecules as a result of thymic positive selection (6, 7). Thus, memory T cells could persist not because they have an inherently longer life-span, but because they receive constant low level stimulation from the same self-peptide/MHC molecules that provide the signaling for positive selection during development. The expression of the self-peptide/MHC complexes that trigger positive selection of CD8+ T cells are largely dependent on the expression of TAP1 (transporter of antigen protein 1) (8, 9). The TAP1 gene encodes an ATP-dependent peptide pump (10), which translocates peptides from the cytosol into the lumen of the endoplasmic reticulum. Peptides then associate with newly synthesized class I heavy chain and β2-microglobulin (β2-m) to form stable class I molecules that are then transported to the cell surface (11–14). In TAP1 mutant mice, this major route of peptide loading of class I molecules is blocked. Consequently, surface expression of MHC class I molecules is reduced to less than 10% of normal levels (8, 15).
In this paper we test the hypothesis that in the absence of antigen, interaction of TCR with self-peptide/MHC molecules is required for the maintenance of CD8+ memory T cells. We analyzed CD8+ T cell memory by using transgenic mice carrying the male-specific, anti-H-Y TCR B6.2.16 (15). The positive selection of B6.2.16 CD8+ cells is dependent on the expression of TAP1-dependent, self-peptide/MHC molecules in the thymus (data not shown). Adoptive transfer of female B6.2.16 CD8+ cells primed with male antigen-presenting cells (APCs) give rise to a population of long-lived memory lymphocytes, which retain the expression of the activation marker CD44 (Pgp-1) and respond vigorously to antigen (16). To eliminate the possibility of transferring persistent antigen in the form of contaminating male cells, the peptide-antigen recognized by B6.2.16 CD8+ cells was identified and this peptide was used to activate female B6.2.16 CD8+ cells in vitro. Activated cells were transferred into female lymphocyte-deficient mice that lacked the expression of recombination activation gene1 (RAG1) (17) and either expressed or lacked TAP1 (8).
The survival of B6.2.16 CD8+ memory cells was drastically impaired in TAP1-deficient hosts. Seventy days after transfer there was a nearly complete absence of anti-H-Y cytotoxic T lymphocyte (CTL) precursors in TAP1− hosts. In addition, the few residual B6.2.16 CD8+ cells in TAP1− hosts had lost the expression of the CD44 activation marker. In contrast, B6.2.16 CD8+ cells transferred into TAP1+ mice survived and gave rise to potent anti-H-Y CTL activity 70 days after adoptive transfer. The heightened reactivity correlates with persistent expression of the CD44 activation marker in the absence of in vivo exposure to either male cells or peptide antigen. Together these data suggest that the activated phenotype and survival of antigen-primed T cells can be maintained in the absence of persistent antigen. The level of TCR engagement provided by self-peptide/MHC complexes found in a positively selecting background appears to be sufficient to maintain the in vivo persistence of memory cells.
MATERIALS AND METHODS
Flow Cytometric Analyses.
The following mAbs were used and purchased from PharMingen: anti-CD4 [R-phycoerythrin (PE) labeled], anti-CD8 [allophycocyanin (APC)], anti-CD44 (PE labeled), and anti-CD62 (PE labeled). The anti-B6.2.16 TCR clonotypic mAb T3.70 (18) (IgG1) (kindly provided by H.-S. Teh, University of British Columbia) was purified from hybridoma supernatant on protein A columns and labeled with fluorescein isothiocynate (FITC) using standard protocols (19). Monovalent Fab fragments of T3.70 mAb were generated, purified, and labeled with FITC using standard protocols (19). Cells were stained and analyzed by fluorescence-activated cell sorting (FACS) using standard protocols.
Mice.
TAP1+RAG1− and TAP1−RAG1− mice (C57BL/6/129Sv) were maintained and bred under pathogen-free conditions. Mice expressing the B6.2.16 TCR α and β transgenes (15) (C57BL/6/129Sv) were maintained and bred under standard conditions.
B6.2.16 Cytotoxic T Cell Assays.
B6.2.16 CTLs were used at an effector-to-target ratio of 10:1 in 51Cr-release assays. RMA cells (20) (H-2b), which do not have a Y chromosome (data not shown), were used as targets. The degree of cytolysis was determined as (experimental release-spontaneous release)/(maximum release-spontaneous release) × 100, in triplicate, in the presence of candidate peptides (10−14 M to 10−8 M).
Purification of Naive B6.2.16 CD8+ Cells.
Mixed spleen and lymph node cell suspensions were prepared from 10–20 female B6.2.16 transgenic mice (4–6 weeks of age); then, nucleated live cells (2–6 × 108) were purified by Ficoll-density centrifugation (Pharmacia). Cell suspensions were depleted of B220+ and CD4+ lymphocytes by magnetic cell sorting with anti-B220 and anti-CD4 beads, then stained with FITC-labeled, T3.70 Fab (15 μg/ml) at a cell concentration of 107/ml, and then B6.2.16+ CD8+ lymphocytes (5–8 × 106) purified by FACS. An aliquot of sorted cells was restained with T3.70-FITC and anti-CD8-PE mAbs, then analyzed by FACS to verify the purity, which was at least 95% B6.2.16 CD8+ cells.
Purification of Activated B6.2.16 CD8+ Cells.
A mixed spleen and lymph node cell suspension was prepared from female B6.2.16 transgenic mice (one or two mice), then cocultured (106 cells per ml, 2 ml per culture) at 37°C with the H-Y peptide (KCSRNRQYL) (10−7 M) in RPMI 1640/10% fetal calf serum. After 3 or 4 days of culture in 24-well plates, live cells were purified by Ficoll-density centrifugation and then stained with FITC-labeled T3.70 Fab (15 μg/ml) at a cell concentration of 107/ml, then B6.2.16 CD8+ lymphocytes (about 5–10 × 106) purified by FACS. The purity was determined to be at least 95% B6.2.16+ CD8+ cells. Purified B6.2.16 CD8+ cells (naive or activated) were resuspended in sterile PBS and then injected into the tail vein (1.5 × 106 per mouse) of 4–6-week-old female RAG1−TAP1+ or RAG1−TAP1− mice (three or four mice in each group).
Determination of the Relative Level of H-2Db on RMA-S Cells.
TAP-deficient RMA-S cells (20), (5 × 105/ml) were incubated for 16 hr with H-Y peptide (2 × 10−4 M) at 37°C in RPMI 1640/10% fetal calf serum. An aliquot of cells (5 × 104) was removed (t = 0) and stained with the anti-H-2Db mAb B22–249R1 (α1-specific, ref. 11) and the level of H-2Db quantitated by FACS. Cells were then washed and incubated at 37°C without peptide. At various time points up to 12 hr, the level of H-2Db surface expression was then determined in triplicate on these cells and on untreated RMA-S and RMA (TAP+) cells. The relative level of surface H-2Db was expressed as the percentage of the RMA level at each time point.
Quantitation of Anti-HY CTL Precursors.
Mixed spleen and lymph node cell suspensions were prepared from RAG1−TAP1+ or RAG1−TAP1− mice 70 days after injection with purified naive or activated B6.2.16 CD8+ cells. To determine the number of B6.2.16 CD8+ cells present in each mouse, an aliquot of cells (5 × 105) was stained with T3.70-FITC, anti-CD8-APC, anti-CD44-PE mAbs and then analyzed by three-color FACS. Limiting dilutions of cells were plated at cell doses from 10 up to 30,000 (24 replicate wells per cell dose) and then cocultured with irradiated female C57BL/6 spleen cells (106), rIL-2 (10 units per ml) in micro-cultures for 7 days. H-Y peptide was added at 10−7 M at the beginning and on day 5 of culture. After 7 days, the micro-cultures were assayed for cytolytic activity to 51Cr-labeled RMA target cells (5 × 103) and H-Y peptide (10−7 M). The cytolytically positive, micro-cultures were defined as those with 51Cr release values exceeding the mean spontaneous release by more than three times the standard deviation. CTL precursor frequency was calculated from the Poisson distribution (19).
RESULTS
Identification of an Epitope Recognized by B6.2.16 Cytotoxic T Cells.
The antigens recognized by both H-2Kk (21) and HLA-B7-restricted (22) anti-H-Y CTLs are derived from the protein encoded by the Y-linked gene Smcy (23). To test the possibility that the anti-H-Y, H-2Db-restricted CTL B6.2.16 (15) also recognizes a peptide from Smcy, the ability of several peptides based on the Smcy protein sequence were tested for the ability to sensitize 51Cr-labeled targets for lysis with B6.2.16 CTLs (Table 1). The Smcy peptides tested have sequences that conform to the H-2Db-specific motif of a nine amino acid peptide with the anchor residue asparagine (N) in the fifth position and either isoleucine, leucine, or methionine (I/L/M) in the ninth position (24). These peptides are: peptide 1 (amino acids 144–152) [PSGKNIGSL], peptide 2 (amino acids 567–575) [VTLMNPNTL], peptide 3 (amino acids 738–746) [KCSRNRQYL], peptide 4 (amino acids 803–811) [RRFPNSELL], peptide 5 (amino acids 1008–1016) [VYLPNIQSL], peptide 6 (amino acids 1345–1353) [ELVLNEERI], peptide 7 (amino acids 1422–1430) [GQNPNLERI]. Peptide 3 was found to be at least five orders of magnitude more effective than any other Smcy peptide at sensitizing H-2b targets to lysis by B6.2.16 CTLs. Thus, peptide 3 is a potent peptide-antigen recognized by B6.2.16 CTLs when presented by H-2Db.
Table 1.
Identification of a male-specific antigen recognized by B6.2.16 CTLs
| Peptide | Sequence | 50max B6.2.16, M |
|---|---|---|
| 1 | PSGKNIGSL | >10−8 |
| 2 | VTLMNPNTL | >10−8 |
| 3 | KCSRNRQYL | 3 × 10−13 |
| 4 | RRFPNSELL | >10−8 |
| 5 | VYLPNIQSL | >10−8 |
| 6 | ELVLNEERI | >10−8 |
| 7 | GQNPNLERI | >10−8 |
The ability of Smcy peptides to sensitize RMA (H-2b) targets to lysis by anti-H-Y B6.2.16 CTLs (15) was tested in 51Cr-release assays and expressed as the concentration of peptide that gave 50% of the maximum specific lysis. The anti-male specificity of the B6.2.16 CTL was demonstrated by measuring the specific lysis of 51Cr-labeled, male (24%) and female (7%) spleen cell blasts from C57BL/6 mice. Similar results were obtained in three other separately performed experiments.
Survival of Naive and Activated CD8+ T Cells.
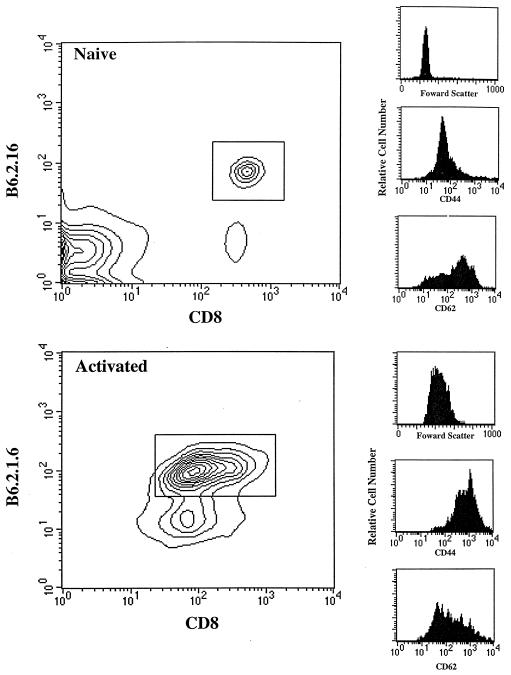
Previous experiments have demonstrated that anti-H-Y, B6.2.16 CD8+ cells stimulated in vivo by introduction into male recipients, give rise to memory cells when adoptively transferred to antigen-free females (16). To eliminate the possibility of transferring persistent antigen in the form of male cells, we tested the possibility of using peptide 3 (hereafter referred to as the H-Y peptide) to generate activated B6.2.16 CD8+ cells in vitro. We cultured lymphocytes from naive female B6.2.16 transgenic mice (15) with the H-Y peptide for 3–4 days at 10−7 M. As shown in Fig. 1, this resulted in the blastogenic activation of the transgenic T cells as evidenced by an increase in the forward light scattering properties of these cells compared with naive cells. The female B6.2.16 CD8+ cells incubated with peptide expressed elevated levels of CD44 (Pgp-1) and lower levels of CD62 (Mel-14) compared with naive cells. Taken together these data indicate that activated B6.2.16 CD8+ cells were generated in vitro by coculture with H-Y peptide.
Figure 1.
Activation of female B6.2.16 T cells by H-Y peptide in vitro. Pooled spleen and mesenteric lymph node cells (5 × 105) from B6.2.16 transgenic mice were stained with the following mAbs: T3.70-FITC [anti-B6.2.16 clonotype (18)], anti-CD8-APC, and either anti-CD44-PE or anti-CD62-PE, and then analyzed by FACS. The expression level of CD44 and CD62 was determined on gated B6.2.16 CD8+ cells. Activated B6.2.16 CD8+ cells were generated by incubating naive cells (106/ml) with the H-Y peptide (10−7 M) for 3–4 days.
Naive B6.2.16 CD8+ cells from female transgenic mice or in vitro activated transgenic T cells were enriched to greater than 95% purity by using a monovalent Fab fragment of the anti-B6.2.16 clonotypic-antibody T3.70, which fails to stimulate proliferation or Ca2+ mobilization in T3.70+ cells (16). Purified naive and activated cells were injected intravenously (1.5 × 106 per mouse) into female RAG1− mice; lymph nodes and spleens were analyzed 10 weeks later.
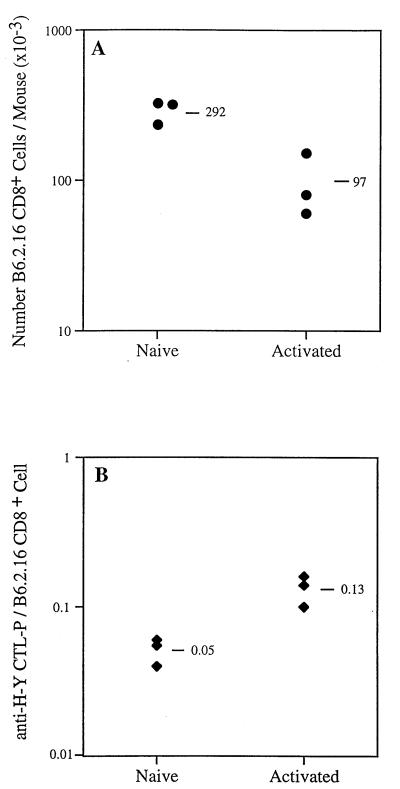
The number of B6.2.16 CD8+ cells recovered from injected RAG1−TAP1+ mice was quantitated by staining with the appropriate mAbs and FACS analysis (as in Fig. 1). As shown in the representative experiment in Fig. 2A, the recovery of naive cells was about 20% of the number of injected; the mean recovery (n = 3) was 292 × 103 B6.2.16 CD8+ cells per mouse. In contrast, the recovery of B6.2.16 CD8+ cells after the same length of time from mice injected with activated cells was about 7% of the number injected; the mean recovery was 97 × 103 B6.2.16 CD8+ cells per mouse. All of the B6.2.16 CD8+ cells recovered from mice injected with activated cells were small lymphocytes, which remained CD44hi even 10 weeks after transfer, whereas naive T cells remained CD44lo (data not shown).
Figure 2.
Survival of naive and activated anti-H-Y T cells in antigen-free mice. (A) The recovery of B6.2.16 CD8+ cells was about three times greater in mice injected with naive compared with activated B6.2.16 CD8+ cells 70 days after the adoptive transfer of 1.5 × 106 purified cells. (B) Limiting dilution analysis was used to determine the frequency of anti-H-Y CTL precursors (anti-H-Y CTL-P) in the recipient RAG1−TAP1+ mice analyzed in A. Similar results have been obtained in two independent experiments.
Although the purified B6.2.16 CD8+ cells were free from persistent antigen in the form of male cells, there was a possibility that H-Y peptide/H-2Db molecules on the surface of the purified, transferred cells could stimulate B6.2.16 CD8+ cells in vivo. To test the likelihood of this, the relative stability of H-Y peptide/H-2Db molecules on RMA-S cells was determined, as described in Materials and Methods. The half-life of H-Y peptide/H-2Db molecules on the surface of RMA-S cells was found to be about 6 hr. Therefore, the potential for stimulation of B6.2.16 CD8+ cells, over the course of 10 weeks, by surface H-Y peptide/H-2Db complexes is limited.
CD8+ T Cells Activated in Vitro Give Rise to Memory T Cells.
The functional capabilities of transferred naive and activated CD8+ T cells were analyzed 10 weeks after injection. By using limiting dilution analysis, we quantitated the number of CTL precursors specific for the H-Y peptide from the lymph nodes and spleens of RAG1−TAP1+ mice 10 weeks after transfer. In the experiment shown in Fig. 2B, the mean number of anti-H-Y CTL precursors derived from each surviving naive B6.2.16 CD8+ cell was 0.05 (mean of three mice), whereas the mean number of anti-HY-CTL precursors derived from each descendant of activated B6.2.16 CD8+ cells was 0.13 (mean of three mice). These data indicate that the small, CD44hi B6.2.16 CD8+ cells derived from activated B6.2.16 CD8+ cells had an enhanced capacity to respond to antigen compared with naive T cells.
TAP1-Dependent Self-Peptide/MHC Molecules Are Required for the Long-Term Survival of CD8+ Memory T Cells.
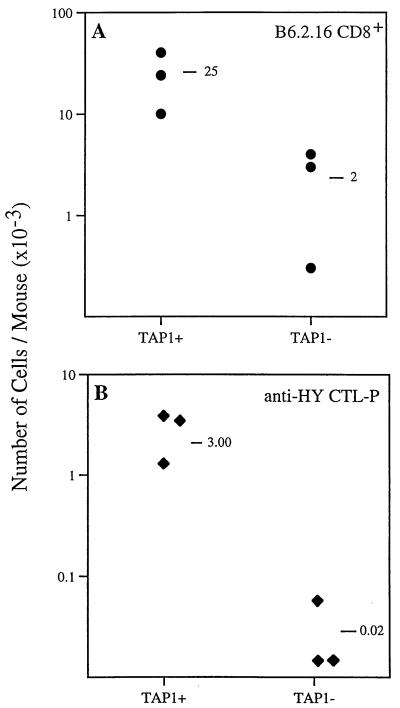
These data demonstrate that memory cells can survive in vivo for at least 10 weeks in the absence of persistent antigen. This survival may reflect the fact that maintenance of memory T cells does not require TCR engagement. However, positive selection ensures that the TCRs expressed by a given T cell have at least a minimal avidity for self-peptide/MHC molecules (25). Thus, the possibility exists that the weak interaction between TCR and self-peptide/MHC may be important in ensuring the survival and functional properties of memory CD8+ T cells. We compared the survival of memory CD8+ cells transferred to mice that express normal self-peptide/MHC class I molecules with those that have impaired expression of self-peptide/MHC class I molecules. We adoptively transferred activated female B6.2.16 CD8+ cells (1.5 × 106 per mouse) to RAG1−TAP1+ and RAG1−TAP1− female mice and determined the number and functional properties of anti-H-Y memory cells after 10 weeks. In the experiment shown in Fig. 3A, the number of B6.2.16 CD8+ cells derived from activated cells adoptively transferred to RAG1−TAP1+ mice (25 × 103 per mouse; mean of three mice) was more than 12 times greater than the number recovered from class I MHClo, RAG1−TAP1− mice (2 × 103 per mouse; mean of three mice).
Figure 3.
Long-term survival of anti-H-Y memory T cells in TAP1-deficient mice. (A) The recovery of memory B6.2.16 CD8+ cells was about 12 times greater in RAG1−TAP1+ compared with RAG1−TAP1− mice (8% of TAP1+ level) 70 days after the adoptive transfer of 1.5 × 106 purified, activated B6.2.16 CD8+ cells. Similar results were obtained in three independent experiments. (B) By using limiting dilution analysis, the frequency of anti-H-Y CTL precursors was determined in the same recipient mice as those analyzed in A. The mean number of anti-H-Y CTL precursors in RAG1−TAP1− mice (0.02 × 103) was less than 1% of the mean number in similarly injected RAG1−TAP1+ mice (3 × 103). Two of the three RAG1−TAP1− mice gave no detectable frequency for anti-H-Y CTL precursors and so are nominally considered to have a frequency of zero. Similar results were obtained in three independent experiments.
The difference in survival of memory cells in TAP1− and TAP1+ mice was even greater when assessed functionally. There was a virtual absence of anti-H-Y CTL precursors recovered from RAG1−TAP1− mice (0.02 × 103 per mouse; mean of three mice) compared with RAG1−TAP1+ mice (3 × 103 per mouse; mean of three mice) (Fig. 3B). The number of anti-H-Y CTL precursors recovered from TAP1− mice after 70 days was only 0.7% (120-fold less) of the number recovered from TAP1+ mice.
Kinetics of Memory CD8+ T Cells’ Survival and Activation.
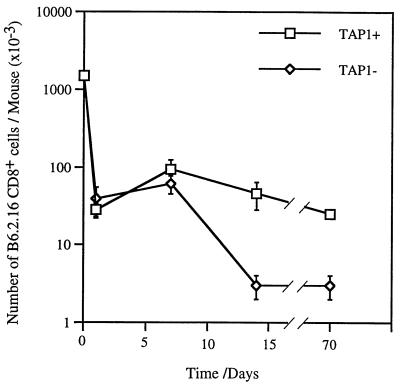
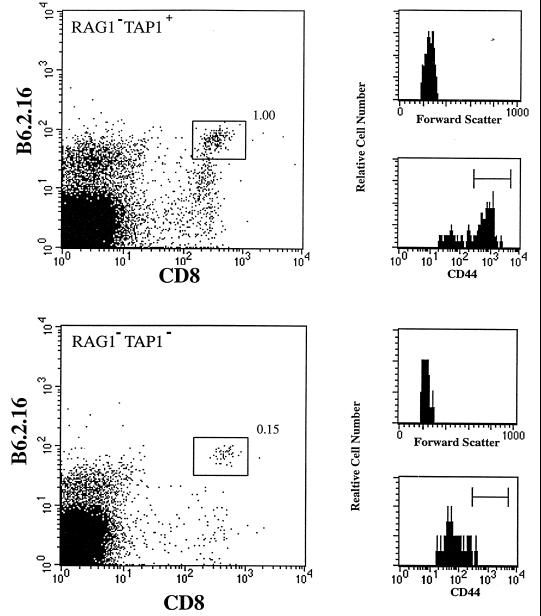
One day after injection only about 5% of the activated B6.2.16 CD8+ cells that were transferred were recovered from either RAG1−TAP1+ or RAG1−TAP1− mice (28 ± 6 × 103 or 39 ± 16 × 103, respectively; mean of three mice). The number of B6.2.16 CD8+ cells in TAP1+ and TAP1− mice 7 days after transfer was also comparable (Fig. 4). There was an increase in the number of CD8+ cells recovered in TAP1+ and TAP1− mice during the first week after transfer. This could be because of class I MHC-independent expansion of transferred CD8+ cells or alternatively could be because of the gradual colonization of the lymph node and spleens of recipient mice by adoptively transferred cells. In addition, 7 days after transfer, the B6.2.16 CD8+ cells recovered from both TAP1+ and TAP1− mice were large and CD44hi (data not shown). In contrast, by 14 days, the number of B6.2.16 CD8+ cells had diminished significantly in TAP1-deficient mice compared with TAP1+ mice (P < 0.1%). At this time point the B6.2.16 CD8+ cells in both TAP1+ and TAP1− mice had reverted to a small cell phenotype, but retained the expression of CD44 (data not shown). Differences in the number of surviving cells persist 70 days after transfer; at this point, the B6.2.16 CD8+ cells lose the expression of CD44 in TAP1− mice but retain it in TAP1+ mice (Fig. 5). These data imply that the expression of TAP1-dependent, self-peptide/MHC molecules is required to ensure both the long term survival and memory phenotype of CD8+ T cells.
Figure 4.
Kinetics of survival of anti-H-Y memory cells. The recovery of memory B6.2.16 CD8+ cells from RAG1−TAP1+ and RAG1−TAP1− mice was determined 1, 7, 14, and 70 days after the adoptive transfer of (t = 0) 1.5 × 106 activated, purified B6.2.16 CD8+ cells. The number of cells at each time point is the mean of 3–4 mice ± SEM.
Figure 5.
Activation status of B6.2.16 T cells in TAP1-deficient mice. The percentage of B6.2.16 CD8+ cells recovered from RAG1−TAP1+ mice 70 days after the adoptive transfer of 1.5 × 106 purified, activated B6.2.16 CD8+ cells was greater (1% of live nucleated cells) than the percentage of the same cells recovered from RAG1−TAP1− mice (0.15%). The B6.2.16 CD8+ cells recovered from RAG1−TAP1+ mice retained the expression of CD44, but the same cells from RAG1−TAP1− mice were CD44lo.
Naive CD8+ T Cells Are Less Dependent on the Surface Expression of TAP1-Dependent Self-Peptide/MHC Molecules than Activated T Cells.
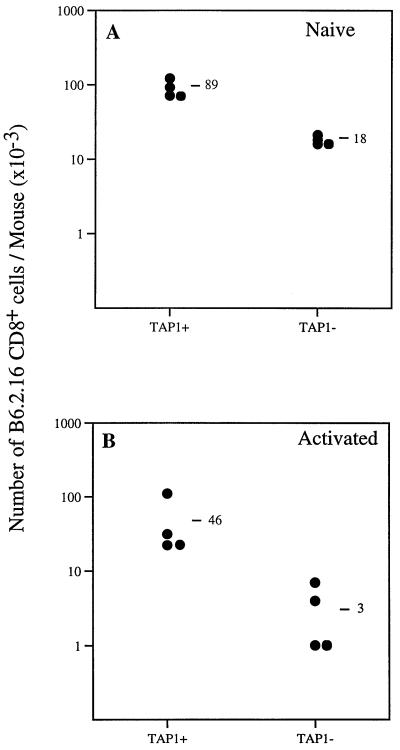
Naive and activated B6.2.16 CD8+ cells were purified and adoptively transferred into RAG1−TAP1+ and RAG1−TAP1− mice. Next, the number of B6.2.16 CD8+ cells was determined after 14 days. The recovery of naive B6.2.16 CD8+ cells was about 5-fold lower in TAP1-deficient mice (18 × 103 cells per mouse; mean of four mice) compared with recipients with normal TAP1 expression (89 × 103 cells per mouse; mean of four mice) (Fig. 6A). The recovery of B6.2.16 CD8+ cells was about 15-fold lower in RAG1−TAP1− mice than in RAG1−TAP1+ mice injected with activated B6.2.16 CD8+ cells (Fig. 6B). These data suggest that naive CD8+ T cells survive better than activated CD8+ T cells in the absence of self-peptide/MHC class I expression.
Figure 6.
Survival of naive and memory anti-H-Y T cells in TAP1-deficient mice. (A) The recovery of naive B6.2.16 CD8+ cells was about five times greater in RAG1−TAP1+ compared with RAG1−TAP1− mice (20% of TAP1+ level) 14 days after adoptive transfer. A similar result has been obtained in another independent experiment. (B) The recovery of memory B6.2.16 CD8+ cells was about 15-fold greater in RAG1−TAP1+ compared with RAG1−TAP1− mice (7% of the RAG1−TAP1+ level) 14 days after adoptive transfer. Similar results have been obtained in two other experiments.
DISCUSSION
Determining which factors generate and maintain memory T cells is critical for our understanding of immunological memory. The essential features of memory T cells are that they are long lived, exhibit an activated phenotype, and show enhanced responsiveness to antigen compared with naive cells (26). The obvious TCR ligand that might maintain such memory T cells is antigen. In the case of viral infections, antigen may well persist over a prolonged period of time, particularly following DNA viral infections (2). However, persistence of antigen is unlikely to explain the long term memory established by immunization with toxoids or killed organisms. In the past it has been suggested that such memory is maintained by cross-reactive environmental antigens (26). Several reports have indicated that under some circumstances (4, 5, 16, 27) memory T cells appear to persist in the absence of antigen. How T cell memory is maintained, in the absence of antigen, has been the focus of our studies.
During T cell development, positive selection requires that the TCRs of developing T cells engage and transmit signals in response to self-peptide/MHC molecules ensuring that mature T cells have specificity for self-MHC and so are capable of MHC-restricted recognition of antigen (6, 7). Therefore, self-peptide/MHC could provide the necessary stimulation to maintain the activated phenotype and survival of memory T cells, in the absence of antigen. To test this hypothesis, we examined the ability of anti-H-Y memory CD8+ T cells to persist in lymphocyte-deficient mice with impaired and normal class I MHC expression. The mean number of cells derived from the adoptively transferred, activated B6.2.16 CD8+ cells recovered after 70 days from class I MHClo (RAG1−TAP1−) mice was only a fraction of the number recovered from mice with normal levels of class I MHC (RAG1−TAP1+) (Fig. 3A). These data indicate that expression of TAP1-dependent, self-peptide/MHC class I complexes is required for the survival of CD8+ memory T cells. When we examined the self-peptide/MHC requirements for the survival of memory cells functionally, we observed a dramatic difference between TAP1+ and TAP1− recipients (Fig. 3B). The mean number of anti-H-Y CTL precursors derived from transferred activated B6.2.16 CD8+ cells recovered after 70 days from TAP1-deficient mice was only 0.7% of the number recovered from mice with normal expression of TAP1 (Fig. 3B). These data indicate that the long-term survival of memory anti-H-Y CTLs is nearly abolished in TAP1-deficient mice.
Previous experiments have shown a less severe reduction in the survival of anti-viral memory CTLs when activated cells were adoptively transferred to β2-m-deficient mice (5). In TAP1− mice, the loading of class I molecules with self-peptides in the lumen of the endoplasmic reticulum is blocked, but unstable “empty” class I molecules can be stabilized at the cell surface by the addition of exogenous peptides (8, 28). In contrast, in β2-m− mice the possibility of a low level of endogenous, self-peptide association with class I heavy chains exists because entry of potential MHC-associated peptides into the lumen of the endoplasmic reticulum is not blocked. Therefore, the repertoire of self-peptides that stabilize the residual class I MHC molecules expressed in TAP1− and β2-m− mice is likely to be different (29). These differences between residual peptide/MHC complexes may explain the differences in survival of CD8+ memory T cells in TAP1− and β2-m− mice.
Over the first 7 days after adoptive transfer, the survival and activation status of B6.2.16 memory cells was the same in RAG1−TAP1+ and RAG1−TAP1− mice (Fig. 4). Therefore, the poor recovery of B6.2.16 CD8+ memory cells in RAG1−TAP1− mice after 70 days was because of impaired survival rather than impaired adoptive transfer. These data, together with the observation that B6.2.16 CD8+ cells are still CD44hi after 14 days in TAP1− mice, imply that the survival and activation of short-term memory cells does not require TCR stimulation by self-peptide/MHC molecules. In contrast, several observations indicate that the long-term survival and activation of memory T cells requires stimulation by self-peptide/MHC molecules. Although we detected residual B6.2.16 CD8+ cells in TAP1− mice (8% of TAP1+ level) 70 days after transfer, in the same hosts we observed a more severe reduction in the numbers of anti-H-Y CTL precursors (0.7% of TAP1+ level) (Fig. 3). This difference implies that the residual B6.2.16 CD8+ cells present in RAG1−TAP1-deficient mice after 70 days had impaired function compared with B6.2.16 CD8+ cells present in mice with normal expression of class I MHC. This interpretation is supported by the second observation that the residual B6.2.16 CD8+ cells present in TAP1− mice after 70 days had a low level of CD44 (Fig. 5). This finding is consistent with our observation that there is a higher degree of naive B6.2.16 CD8+ cell survival in TAP1-deficient mice (5-fold lower than TAP1+ mice) compared with memory B6.2.16 CD8+ survival in TAP1-deficient mice (15-fold lower than TAP1+ mice) (Fig. 6). We conclude, therefore, that activated memory T cells are more dependent on self-peptide/MHC molecules than naive cells for their long-term survival.
During the preparation of this paper it was reported that β2-m expression was required for the short-term (16 days) survival of cells derived from activated CD8+ cells, some of which are presumably destined to become memory cells (27). Our observations confirm this finding by using TAP1-deficient mice and demonstrate that cross-reactive interactions between the anti-H-Y TCR of CD8+ T cells and TAP1-dependent, self-peptide/class I MHC molecules are essential to ensure the long-term survival of memory T cells, in the absence of antigen. Tanchot et al. (27) observed in vivo expansion of memory CD8+ cells during the first few weeks after transfer. However, those experiments differ from these reported here because Tanchot et al. activated B6.2.16 CD8+ cells by adoptive transfer in male mice. Therefore, the in vivo expansion of activated cells, after transfer to female mice, may have been stimulated by contaminating male cell-antigen (potentially up to 3 × 104 cells in each preparation). This may be why, in contrast to our findings (Fig. 4) and that of others (16), Tanchot et al. did not observe a decrease in anti-H-Y CD8+ cell number after adoptive transfer, which is presumably because of activation induced cell death (30). The less stringent requirements of memory B6.2.16 CD8+ cells, for in vivo survival compared with naive cells, in normal and β2-m− mice, observed by Tanchot et al., may also be because of in vivo antigenic stimulation by contaminating persistent antigen in the form of male cells.
During positive selection, only thymocytes that express TCRs with the appropriate avidity for self-peptide/MHC are given the signal(s) necessary to avoid programmed cell death and differentiate into mature T cells (25, 31, 32). Therefore, a potential role of positive selection may be to ensure that mature T cells express TCRs that are weakly reactive to self-peptide/MHC complexes, thereby providing memory cells with a low level of stimulation to ensure long-term survival in the absence of antigen. Although the survival of naive CD4+ T cells (33) and naive CD8+ T cells (27) require interaction of TCRs with self-peptide/MHC molecules for survival, we observed that memory T cells are more dependent than naive cells on these low avidity signals for their long-term survival.
Acknowledgments
We thank Julie Auger and Paul Butterfield of The University of Chicago Flow Cytometry Core Facility for cell sorting. This facility is supported by grants from the National Cancer Institute (CA-14599) and National Institute of Diabetes and Digestive and Kidney Diseases (PO1-DK49799).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: MHC, major histocompatibility complex; TCR, T cell receptor; TAP1, transporter of antigen protein 1; β2-m, β2-microglobulin; CTL, cytotoxic T cell; RAG1, recombination activation gene 1; FITC, fluorescein isothiocynate; FACS, fluorescence-activated cell sorting.
References
- 1.Gray D, Matzinger P. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oehen S, Waldner H, Kundig T M, Hengartner H, Zinkernagel R. J Exp Med. 1992;176:1273–1281. doi: 10.1084/jem.176.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullbacher A. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Nature (London) 1994;369:648–652. [Google Scholar]
- 5.Hou S, Hyland L, Ryan K W, Portner A, Doherty P C. Nature (London) 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 6.Zinkernagel R M, Doherty P C. Nature (London) 1974;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- 7.Bevan M J. Nature (London) 1977;269:417–418. [Google Scholar]
- 8.Van Kaer L, Ashton-Rickardt P G, Ploegh H L, Tonegawa S. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 9.Ashton-Rickardt P G, Van Kaer L, Schumacher T N M, Ploegh H L, Tonegawa S. Cell. 1993;73:1041–1049. doi: 10.1016/0092-8674(93)90281-t. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd J C, Schumacher T N M, Ashton-Rickardt P G, Imaeda S, Ploegh H L, Janeway C A J, Tonegawa S. Cell. 1993;74:577–584. doi: 10.1016/0092-8674(93)80058-m. [DOI] [PubMed] [Google Scholar]
- 11.Townsend A, Ohlen C, Bastin J, Ljunggren H-G, Foster L, Karre K. Nature (London) 1989;340:443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 12.Townsend A, Elliott J, Cerundolo V, Foster L, Barber B, Tse A. Cell. 1990;62:285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- 13.Nuchtern J G, Bonifacino J S, Biddison W E, Klausner R D. Nature (London) 1989;339:223–226. doi: 10.1038/339223a0. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher T N M, Heemels M-T, Neefjes J J, Kast W M, Melief C J M, Ploegh H L. Cell. 1990;62:563–567. doi: 10.1016/0092-8674(90)90020-f. [DOI] [PubMed] [Google Scholar]
- 15.Kisielow P, Bluthmann H, Staerz U D, Steinmetz M, von Boehmer H. Nature (London) 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 16.Bruno L, Kirberg J, von Boehmer H. Immunity. 1995;2:37–43. doi: 10.1016/1074-7613(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 17.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 18.Teh H S, Kishi H, Scott B, Von Boehmer H. J Exp Med. 1989;169:795–806. doi: 10.1084/jem.169.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coligan J E, Kruisbeek A M, Margulis D H, Shevach E H, Straber W. Current Protocols in Immunology. New York: Wiley; 1995. [Google Scholar]
- 20.Ljunggren H-G, Karre K. J Exp Med. 1985;162:1745–1754. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott D M, Ehrmann I E, Ellis P S, Bishop C E, Agulnik A I, Simpson E, Mitchell M J. Nature (London) 1995;376:695–698. doi: 10.1038/376695a0. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Meadows L R, den Haan J M M, Sherman N E, Chen Y, Blokland E, Shabanowitz J, Agulnik A I, Henderson R C, Bishop C E, et al. Science. 1995;269:1588–1590. doi: 10.1126/science.7667640. [DOI] [PubMed] [Google Scholar]
- 23.Agulnik A I, Mitchell M J, Lerner J L, Bishop C E. Hum Mol Genet. 1994;3:873–878. doi: 10.1093/hmg/3.6.873. [DOI] [PubMed] [Google Scholar]
- 24.Falk K, Rotzachke O, Stevanivic O, Jung G, Rammensee H-G. Nature (London) 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 25.Ashton-Rickardt P G, Bandeira A, Delaney J R, Van Kaer L, Pircher H-P, Zinkernagel R M, Tonegawa S. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 26.Beverley P C L. Immunol Today. 1990;11:203–205. doi: 10.1016/0167-5699(90)90083-l. [DOI] [PubMed] [Google Scholar]
- 27.Tanchot C, Lemonnier F A, Perarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 28.Ljunggren H-G, Stam N J, Ohlen C, Neefjes J J, Hoglund P, Heemels M-T, Bastin J, Schumacher T N M, Townsend A, Karre K, Ploegh H L. Nature (London) 1990;346:476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 29.Ljunggren H-G, Van Kaer L, Sabatine M S, Auchincloss H, Jr, Tonegawa S, Ploegh H L. Int Immunol. 1995;7:975–984. doi: 10.1093/intimm/7.6.975. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed R, Gray D. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 31.Hu Q, Bazemore Walker C R, Girao C, Opferman J T, Sun J, Shabanowitz J, Hunt D F, Ashton-Rickardt P G. Immunity. 1997;7:221–231. doi: 10.1016/s1074-7613(00)80525-7. [DOI] [PubMed] [Google Scholar]
- 32.Hogquist K A, Tomlinson A J, Keiper W C, McGargill M A, Hart M C, Naylor S, Jameson S C. Immunity. 1997;6:389–399. doi: 10.1016/s1074-7613(00)80282-4. [DOI] [PubMed] [Google Scholar]
- 33.Takeda S, Rodewald H-R, Arakawa H, Bluthmann H, Shimizu T. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]