Abstract
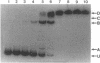
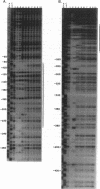
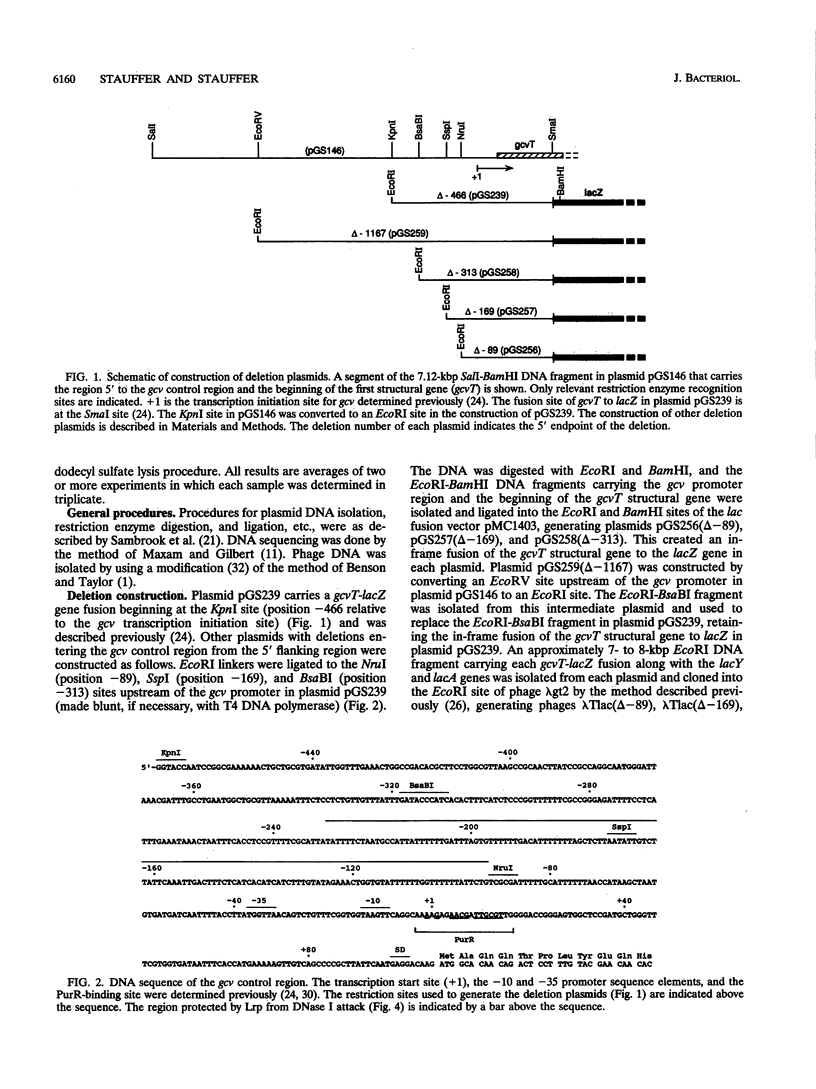
We constructed a set of deletions upstream of the gcv promoter and analyzed the effects of the deletions on expression of a gcvT-lacZ gene fusion. A deletion that ends at position -313 upstream of the transcription initiation site (+1) results in reduced levels of gcvT-lacZ expression, but the fusion is still inducible by glycine and repressible by purines. A deletion that ends at position -169 results in loss of both GcvA- and Lrp-mediated activation of the gcvT-lacZ fusion. The endpoints of delta -313 and delta -169 also define a site that down-regulates gcvT-lacZ expression two- to threefold. A deletion that ends at position -89 upstream from the transcription initiation site still shows PurR-mediated repression, suggesting that PurR-mediated repression is not by direct interference with the GcvA- and Lrp-mediated regulatory mechanism(s). Gel mobility shift assays and DNase I footprinting showed that Lrp protein binds to multiple sites upstream of the gcv promoter, from about bp -92 to bp -229. The results suggest that the gcv regulatory region is complex, with numerous cis-acting sites that are required for normal gcv expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernsting B. R., Denninger J. W., Blumenthal R. M., Matthews R. G. Regulation of the gltBDF operon of Escherichia coli: how is a leucine-insensitive operon regulated by the leucine-responsive regulatory protein? J Bacteriol. 1993 Nov;175(22):7160–7169. doi: 10.1128/jb.175.22.7160-7169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973 Jun 27;1(2):169–187. doi: 10.1007/BF01659328. [DOI] [PubMed] [Google Scholar]
- Kilstrup M., Meng L. M., Neuhard J., Nygaard P. Genetic evidence for a repressor of synthesis of cytosine deaminase and purine biosynthesis enzymes in Escherichia coli. J Bacteriol. 1989 Apr;171(4):2124–2127. doi: 10.1128/jb.171.4.2124-2127.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., D'Ari R., Newman E. B. Lambda placMu insertions in genes of the leucine regulon: extension of the regulon to genes not regulated by leucine. J Bacteriol. 1992 Mar;174(6):1948–1955. doi: 10.1128/jb.174.6.1948-1955.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meedel T. H., Pizer L. I. Regulation of one-carbon biosynthesis and utilization in Escherichia coli. J Bacteriol. 1974 Jun;118(3):905–910. doi: 10.1128/jb.118.3.905-910.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura-Ikeda K., Ohmura Y., Fujiwara K., Motokawa Y. Cloning and nucleotide sequence of the gcv operon encoding the Escherichia coli glycine-cleavage system. Eur J Biochem. 1993 Sep 1;216(2):539–548. doi: 10.1111/j.1432-1033.1993.tb18172.x. [DOI] [PubMed] [Google Scholar]
- Panasenko S. M., Cameron J. R., Davis R. W., Lehman I. R. Five hundredfold overproduction of DNA ligase after induction of a hybrid lambda lysogen constructed in vitro. Science. 1977 Apr 8;196(4286):188–189. doi: 10.1126/science.322281. [DOI] [PubMed] [Google Scholar]
- Rex J. H., Aronson B. D., Somerville R. L. The tdh and serA operons of Escherichia coli: mutational analysis of the regulatory elements of leucine-responsive genes. J Bacteriol. 1991 Oct;173(19):5944–5953. doi: 10.1128/jb.173.19.5944-5953.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfes R. J., Zalkin H. Escherichia coli gene purR encoding a repressor protein for purine nucleotide synthesis. Cloning, nucleotide sequence, and interaction with the purF operator. J Biol Chem. 1988 Dec 25;263(36):19653–19661. [PubMed] [Google Scholar]
- Rolfes R. J., Zalkin H. Regulation of Escherichia coli purF. Mutations that define the promoter, operator, and purine repressor gene. J Biol Chem. 1988 Dec 25;263(36):19649–19652. [PubMed] [Google Scholar]
- SAGERS R. D., GUNSALUS I. C. Intermediatry metabolism of Diplococcus glycinophilus. I. Glycine cleavage and one-carbon interconversions. J Bacteriol. 1961 Apr;81:541–549. doi: 10.1128/jb.81.4.541-549.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Stauffer L. T., Ghrist A., Stauffer G. V. The Escherichia coli gcvT gene encoding the T-protein of the glycine cleavage enzyme system. DNA Seq. 1993;3(6):339–346. doi: 10.3109/10425179309020835. [DOI] [PubMed] [Google Scholar]
- Stauffer L. T., Plamann M. D., Stauffer G. V. Cloning and characterization of the glycine-cleavage enzyme system of Escherichia coli. Gene. 1986;44(2-3):219–226. doi: 10.1016/0378-1119(86)90185-x. [DOI] [PubMed] [Google Scholar]
- Urbanowski M. L., Plamann L. S., Stauffer G. V. Mutations affecting the regulation of the metB gene of Salmonella typhimurium LT2. J Bacteriol. 1987 Jan;169(1):126–130. doi: 10.1128/jb.169.1.126-130.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wang Q., Calvo J. M. Lrp, a global regulatory protein of Escherichia coli, binds co-operatively to multiple sites and activates transcription of ilvIH. J Mol Biol. 1993 Jan 20;229(2):306–318. doi: 10.1006/jmbi.1993.1036. [DOI] [PubMed] [Google Scholar]
- Wilson R. L., Stauffer L. T., Stauffer G. V. Roles of the GcvA and PurR proteins in negative regulation of the Escherichia coli glycine cleavage enzyme system. J Bacteriol. 1993 Aug;175(16):5129–5134. doi: 10.1128/jb.175.16.5129-5134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. L., Steiert P. S., Stauffer G. V. Positive regulation of the Escherichia coli glycine cleavage enzyme system. J Bacteriol. 1993 Feb;175(3):902–904. doi: 10.1128/jb.175.3.902-904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]