Abstract
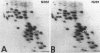
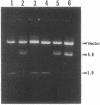
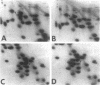
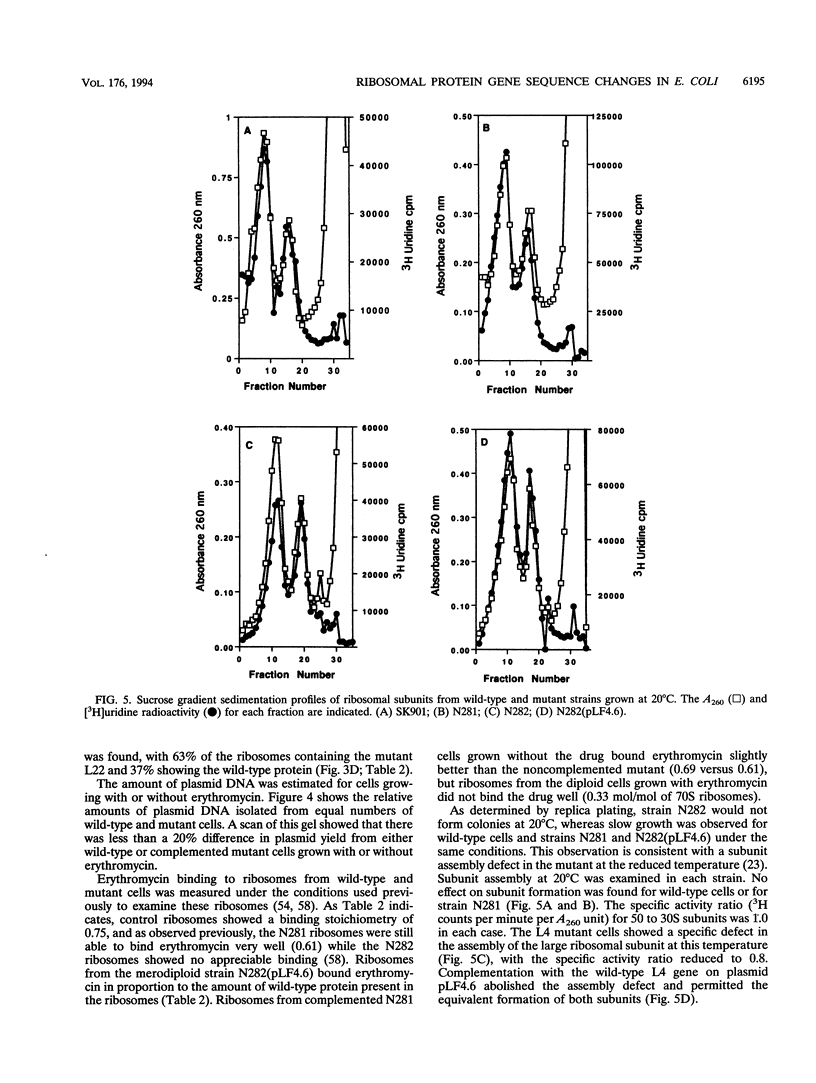
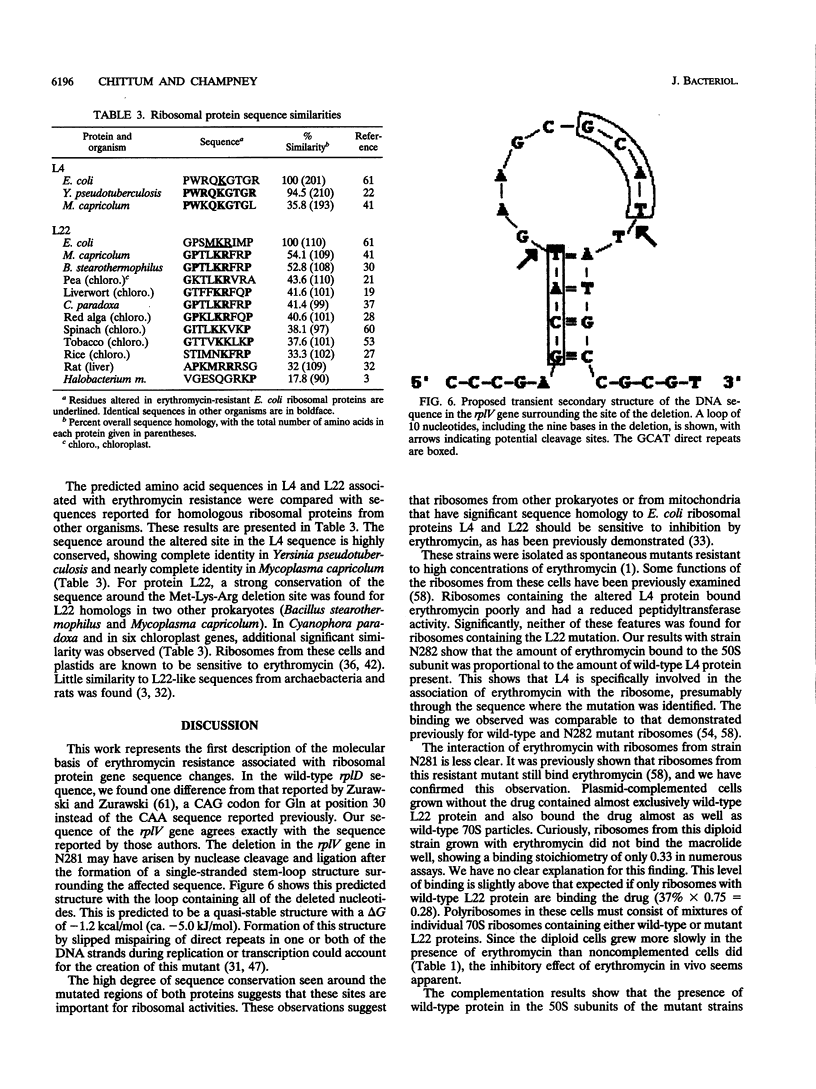
The genes for ribosomal proteins L4 and L22 from two erythromycin-resistant mutants of Escherichia coli have been isolated and sequenced. In the L4 mutant, an A-to-G transition in codon 63 predicted a Lys-to-Glu change in the protein. In the L22 strain, a 9-bp deletion removed codons 82 to 84, eliminating the sequence Met-Lys-Arg from the protein. Consistent with these DNA changes, in comparison with wild-type proteins, both mutant proteins had reduced first-dimension mobilities in two-dimensional polyacrylamide gels. Complementation of each mutation by a wild-type gene on a plasmid vector resulted in increased erythromycin sensitivity in the partial-diploid strains. The fraction of ribosomes containing the mutant form of the protein was increased by growth in the presence of erythromycin. Erythromycin binding was increased by the fraction of wild-type protein present in the ribosome population. The strain with the L4 mutation was found to be cold sensitive for growth at 20 degrees C, and 50S-subunit assembly was impaired at this temperature. The mutated sequences are highly conserved in the corresponding proteins from a number of species. The results indicate the participation of these proteins in the interaction of erythromycin with the ribosome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D. Three genes that affect Escherichia coli ribosomes. J Mol Biol. 1967 Dec 14;30(2):255–275. [PubMed] [Google Scholar]
- Arndt E., Krömer W., Hatakeyama T. Organization and nucleotide sequence of a gene cluster coding for eight ribosomal proteins in the archaebacterium Halobacterium marismortui. J Biol Chem. 1990 Feb 25;265(6):3034–3039. [PubMed] [Google Scholar]
- Arévalo M. A., Tejedor F., Polo F., Ballesta J. P. Protein components of the erythromycin binding site in bacterial ribosomes. J Biol Chem. 1988 Jan 5;263(1):58–63. [PubMed] [Google Scholar]
- Barritault D., Expert-Bezancon A., Guérin M. F., Hayes D. The use of acetone precipitation in the isolation of ribosomal proteins. Eur J Biochem. 1976 Mar 16;63(1):131–135. doi: 10.1111/j.1432-1033.1976.tb10215.x. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Champney W. S. Localized mutagenesis for the isolation of temperature-sensitive mutants of Escherichia coli affected in protein synthesis. Genetics. 1979 Feb;91(2):215–227. doi: 10.1093/genetics/91.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champney W. S. Protein synthesis defects in temperature-sensitive mutants of Escherichia coli with altered ribosomal proteins. Biochim Biophys Acta. 1980 Oct 17;609(3):464–474. doi: 10.1016/0005-2787(80)90120-3. [DOI] [PubMed] [Google Scholar]
- Chinali G., Nyssen E., Di Giambattista M., Cocito C. Action of erythromycin and virginiamycin S on polypeptide synthesis in cell-free systems. Biochim Biophys Acta. 1988 Nov 10;951(1):42–52. doi: 10.1016/0167-4781(88)90023-1. [DOI] [PubMed] [Google Scholar]
- Cundliffe E. On the nature of antibiotic binding sites in ribosomes. Biochimie. 1987 Aug;69(8):863–869. doi: 10.1016/0300-9084(87)90213-6. [DOI] [PubMed] [Google Scholar]
- Douthwaite S. Functional interactions within 23S rRNA involving the peptidyltransferase center. J Bacteriol. 1992 Feb;174(4):1333–1338. doi: 10.1128/jb.174.4.1333-1338.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douthwaite S., Prince J. B., Noller H. F. Evidence for functional interaction between domains II and V of 23S ribosomal RNA from an erythromycin-resistant mutant. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8330–8334. doi: 10.1073/pnas.82.24.8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettayebi M., Prasad S. M., Morgan E. A. Chloramphenicol-erythromycin resistance mutations in a 23S rRNA gene of Escherichia coli. J Bacteriol. 1985 May;162(2):551–557. doi: 10.1128/jb.162.2.551-557.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa H., Kohchi T., Sano T., Shirai H., Umesono K., Inokuchi H., Ozeki H., Ohyama K. Structure and organization of Marchantia polymorpha chloroplast genome. III. Gene organization of the large single copy region from rbcL to trnI(CAU). J Mol Biol. 1988 Sep 20;203(2):333–351. doi: 10.1016/0022-2836(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Gantt J. S., Baldauf S. L., Calie P. J., Weeden N. F., Palmer J. D. Transfer of rpl22 to the nucleus greatly preceded its loss from the chloroplast and involved the gain of an intron. EMBO J. 1991 Oct;10(10):3073–3078. doi: 10.1002/j.1460-2075.1991.tb07859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross U., Chen J. H., Kono D. H., Lobo J. G., Yu D. T. High degree of conservation between ribosomal proteins of Yersinia pseudotuberculosis and Escherichia coli. Nucleic Acids Res. 1989 May 11;17(9):3601–3602. doi: 10.1093/nar/17.9.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampl H., Schulze H., Nierhaus K. H. Ribosomal components from Escherichia coli 50 S subunits involved in the reconstitution of peptidyltransferase activity. J Biol Chem. 1981 Mar 10;256(5):2284–2288. [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Herold M., Nierhaus K. H. Incorporation of six additional proteins to complete the assembly map of the 50 S subunit from Escherichia coli ribosomes. J Biol Chem. 1987 Jun 25;262(18):8826–8833. [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Kao J. S., Wu M. The sequence of the plastid encoded rpl22 protein in marine macroalgae, Gracilaria tenuistipitata. Nucleic Acids Res. 1990 May 25;18(10):3067–3067. doi: 10.1093/nar/18.10.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny J. W., Lambert J. M., Traut R. R. Cross-linking of ribosomes using 2-iminothiolane (methyl 4-mercaptobutyrimidate) and identification of cross-linked proteins by diagonal polyacrylamide/sodium dodecyl sulfate gel electrophoresis. Methods Enzymol. 1979;59:534–550. doi: 10.1016/0076-6879(79)59112-5. [DOI] [PubMed] [Google Scholar]
- Krömer W. J., Hatakeyama T., Kimura M. Nucleotide sequences of Bacillus stearothermophilus ribosomal protein genes: part of the ribosomal S10 operon. Biol Chem Hoppe Seyler. 1990 Jul;371(7):631–636. doi: 10.1515/bchm3.1990.371.2.631. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Misalignment-mediated DNA synthesis errors. Biochemistry. 1990 Sep 4;29(35):8003–8011. doi: 10.1021/bi00487a001. [DOI] [PubMed] [Google Scholar]
- Laine R. O., Laipis P. J., Shay N. F., Kilberg M. S. Identification of an amino acid-regulated mRNA from rat liver as the mammalian equivalent of bacterial ribosomal protein L22. J Biol Chem. 1991 Sep 15;266(26):16969–16972. [PubMed] [Google Scholar]
- Lamb A. J., Clark-Walker G. D., Linnane A. W. The biogenesis of mitochondria. 4. The differentiation of mitochondrial and cytoplasmic protein synthesizing systems in vitro by antibiotics. Biochim Biophys Acta. 1968 Jul 23;161(2):415–427. [PubMed] [Google Scholar]
- Le Grice S. F., Matzura H. Binding of RNA polymerase and the catabolite gene activator protein within the cat promoter in Escherichia coli. J Mol Biol. 1981 Aug 5;150(2):185–196. doi: 10.1016/0022-2836(81)90448-4. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Putterman M. The intermolecular complex of erythromycin and ribosome. J Mol Biol. 1969 Sep 14;44(2):347–361. doi: 10.1016/0022-2836(69)90180-6. [DOI] [PubMed] [Google Scholar]
- Mets L. J., Bogorad L. Mendelian and uniparental alterations in erythromycin binding by plastid ribosomes. Science. 1971 Nov 12;174(4010):707–709. doi: 10.1126/science.174.4010.707. [DOI] [PubMed] [Google Scholar]
- Michalowski C. B., Pfanzagl B., Löffelhardt W., Bohnert H. J. The cyanelle S10 spc ribosomal protein gene operon from Cyanophora paradoxa. Mol Gen Genet. 1990 Nov;224(2):222–231. doi: 10.1007/BF00271555. [DOI] [PubMed] [Google Scholar]
- Nomura M., Engbaek F. Expression of ribosomal protein genes as analyzed by bacteriophage Mu-induced mutations. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1526–1530. doi: 10.1073/pnas.69.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo S., Muto A., Kawauchi Y., Yamao F., Osawa S. The ribosomal protein gene cluster of Mycoplasma capricolum. Mol Gen Genet. 1987 Dec;210(2):314–322. doi: 10.1007/BF00325700. [DOI] [PubMed] [Google Scholar]
- Pardo D., Rosset R. A new ribosomal mutation which affects the two ribosomal subunits in Escherichia coli. Mol Gen Genet. 1977 Jun 8;153(2):199–204. doi: 10.1007/BF00264736. [DOI] [PubMed] [Google Scholar]
- Pardo D., Rosset R. Genetic studies of erythromycin resistant mutants of Escherichia coli. Mol Gen Genet. 1974;135(3):257–268. doi: 10.1007/BF00268620. [DOI] [PubMed] [Google Scholar]
- Pardo D., Rosset R. Properties of ribosomes from erythromycin resistant mutants of Escherichia coli. Mol Gen Genet. 1977 Nov 18;156(3):267–271. doi: 10.1007/BF00267181. [DOI] [PubMed] [Google Scholar]
- Pestka S. Binding of [14C]erythromycin to Escherichia coli ribosomes. Antimicrob Agents Chemother. 1974 Oct;6(4):474–478. doi: 10.1128/aac.6.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley L. S. Model for the participation of quasi-palindromic DNA sequences in frameshift mutation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4128–4132. doi: 10.1073/pnas.79.13.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnier J., Gewitz H. S., Behrens S. E., Lee A., Ginther C., Leighton T. Isolation and characterization of Bacillus stearothermophilus 30S and 50S ribosomal protein mutations. J Bacteriol. 1990 Dec;172(12):7306–7309. doi: 10.1128/jb.172.12.7306-7309.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock R. A., Leighton T., Wittmann H. G. Macrolide and aminoglycoside antibiotic resistance mutations in the bacillus subtilis ribosome resulting in temperature-sensitive sporulation. Mol Gen Genet. 1981;183(3):538–543. doi: 10.1007/BF00268778. [DOI] [PubMed] [Google Scholar]
- Sigmund C. D., Morgan E. A. Erythromycin resistance due to a mutation in a ribosomal RNA operon of Escherichia coli. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5602–5606. doi: 10.1073/pnas.79.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Davis B. D. Action of antibiotics on chain-initiating and on chain-elongating ribosomes. Methods Enzymol. 1979;59:851–862. doi: 10.1016/0076-6879(79)59130-7. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Wakasugi T., Sugita M., Shinozaki K., Sugiura M. Genes for the eight ribosomal proteins are clustered on the chloroplast genome of tobacco (Nicotiana tabacum): similarity to the S10 and spc operons of Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6030–6034. doi: 10.1073/pnas.83.16.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka H. A reversible change in the ability of Escherichia coli ribosomes to bind to erythromycin. J Mol Biol. 1970 Mar;48(3):511–515. doi: 10.1016/0022-2836(70)90062-8. [DOI] [PubMed] [Google Scholar]
- Teraoka H., Nierhaus K. H. Proteins fro Escherichia coli ribosomes involved in the binding of erythromycin. J Mol Biol. 1978 Dec 5;126(2):185–193. doi: 10.1016/0022-2836(78)90358-3. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Johnson C. W., Ginther C. L., Leighton T., Wittmann H. G. Erythromycin resistant mutations in Bacillus subtilis cause temperature sensitive sporulation. Mol Gen Genet. 1977 Jan 18;150(2):147–159. doi: 10.1007/BF00695395. [DOI] [PubMed] [Google Scholar]
- Wittmann H. G., Stöffler G., Apirion D., Rosen L., Tanaka K., Tamaki M., Takata R., Dekio S., Otaka E. Biochemical and genetic studies on two different types of erythromycin resistant mutants of Escherichia coli with altered ribosomal proteins. Mol Gen Genet. 1973 Dec 20;127(2):175–189. doi: 10.1007/BF00333665. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Lindahl L. Domain I of 23S rRNA competes with a paused transcription complex for ribosomal protein L4 of Escherichia coli. Nucleic Acids Res. 1993 May 25;21(10):2429–2435. doi: 10.1093/nar/21.10.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. X., Quigley F., Massenet O., Mache R. Cotranscription of the S10- and spc-like operons in spinach chloroplasts and identification of three of their gene products. Mol Gen Genet. 1989 Apr;216(2-3):439–445. doi: 10.1007/BF00334388. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Zurawski S. M. Structure of the Escherichia coli S10 ribosomal protein operon. Nucleic Acids Res. 1985 Jun 25;13(12):4521–4526. doi: 10.1093/nar/13.12.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]