Abstract
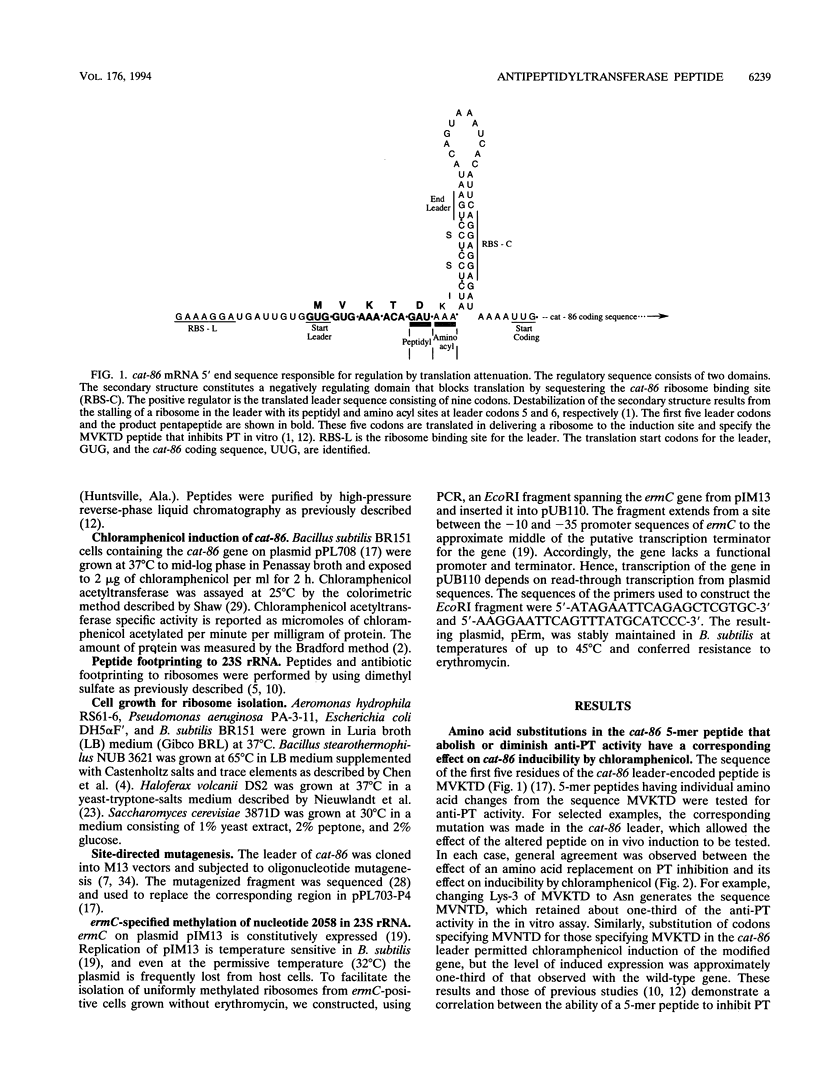
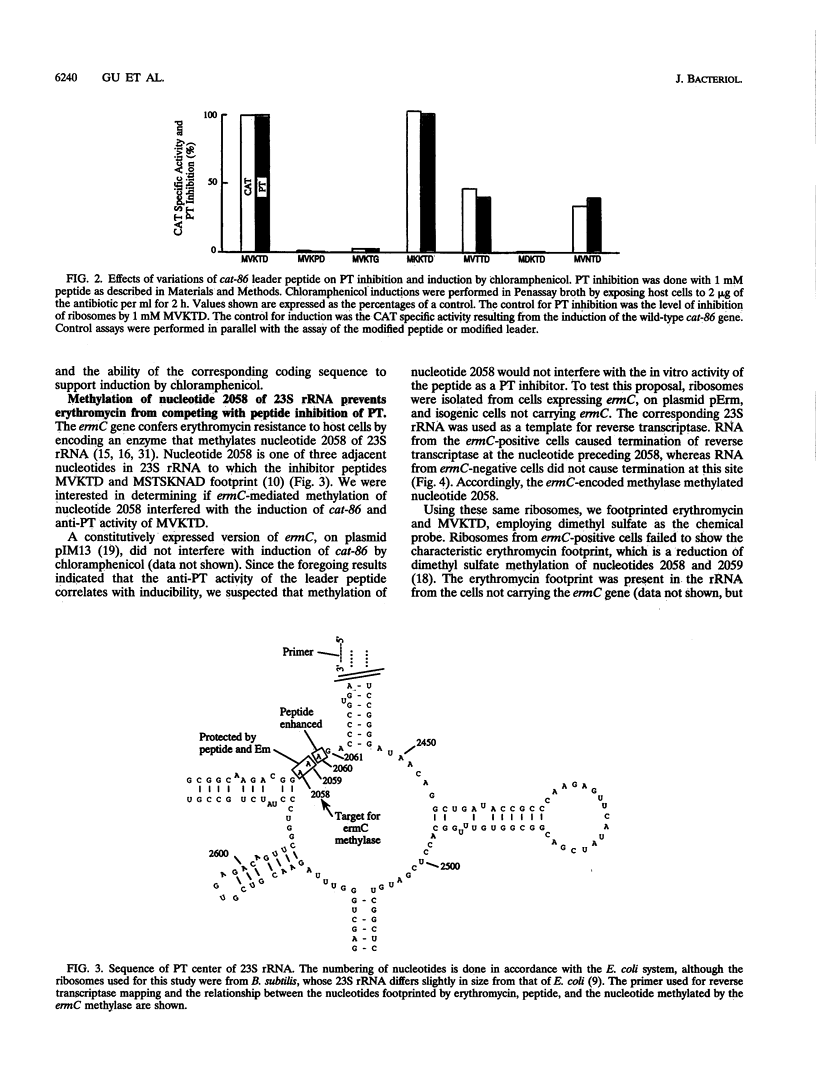
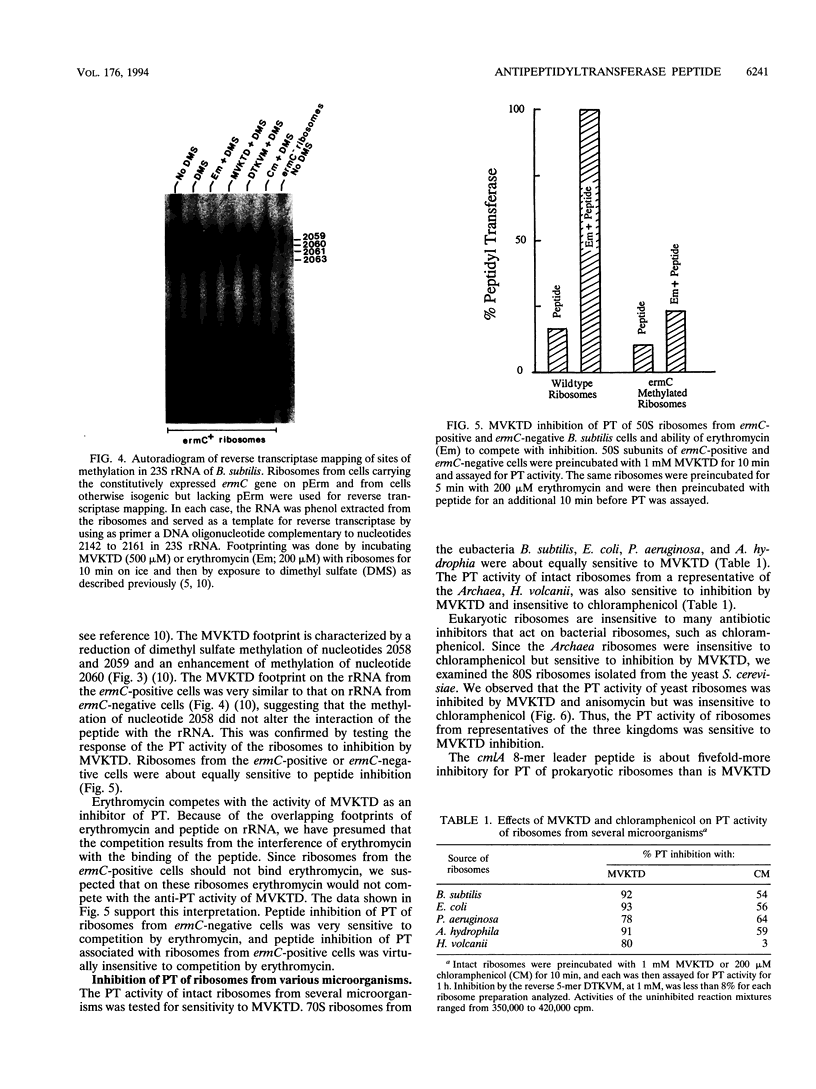
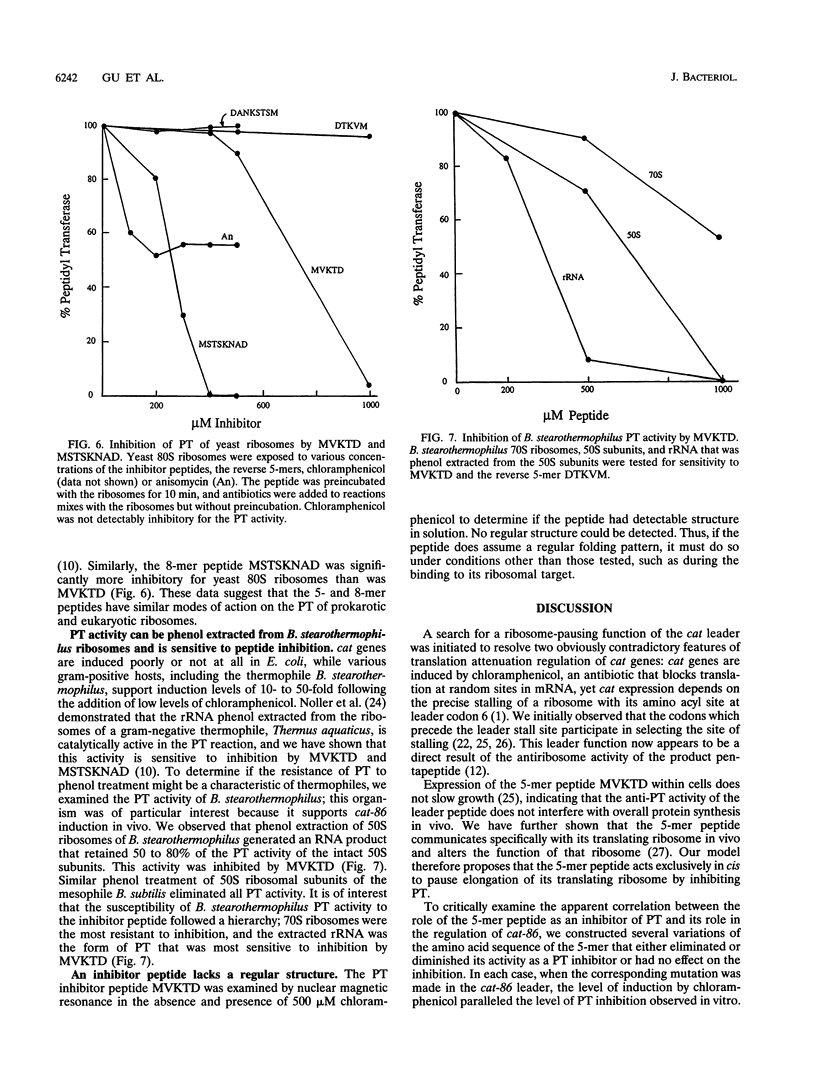
Inducible chloramphenicol resistance genes cat and cmlA are regulated by translation attenuation. For both genes, the leader codons that must be translated to deliver a ribosome to the induction site specify a peptide that inhibits peptidyltransferase in vitro. The antipeptidyltransferase activity of the peptides is thought to select the site of ribosome stalling that is essential for induction. Using variations of the cat-86 leader-encoded 5-mer peptide MVKTD, we demonstrate a correlation between the in vitro antipeptidyltransferase activity and the ability of the same peptide to support induction by chloramphenicol in vivo. MVKTD footprints to nucleotides 2058, 2059, and 2060 in 23S rRNA. In vivo methylation of nucleotide 2058 by the ermC methylase interferes neither with cat-86 induction nor with peptide inhibition of peptidyltransferase. The methylation eliminates the competition that normally occurs in vitro between erythromycin and MVKTD. MVKTD inhibits the peptidyltransferase of several eubacteria, a representative Archaea species, and the eukaryote Saccharomyces cerevisiae. Bacillus stearothermophilus supports the in vivo induction of cat-86, and the RNA that is phenol extracted from the 50S ribosomes of this gram-positive thermophile is catalytically active in the peptidyltransferase assay and sensitive to peptide inhibition. Our results indicate that peptidyltransferase inhibition by a cat leader peptide is essential to induction, and this activity can be altered by minor changes in the amino acid sequence of the peptide. The broad range of organisms shown to possess peptide-inhibitable peptidyltransferase suggests that the target is a highly conserved component of the ribosome and includes 23S rRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexieva Z., Duvall E. J., Ambulos N. P., Jr, Kim U. J., Lovett P. S. Chloramphenicol induction of cat-86 requires ribosome stalling at a specific site in the leader. Proc Natl Acad Sci U S A. 1988 May;85(9):3057–3061. doi: 10.1073/pnas.85.9.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner R., Matzura H. Regulation of the inducible chloramphenicol acetyltransferase gene of the Staphylococcus aureus plasmid pUB112. EMBO J. 1985 Sep;4(9):2295–2300. doi: 10.1002/j.1460-2075.1985.tb03929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. F., Wojcik S. F., Welker N. E. Genetic analysis of Bacillus stearothermophilus by protoplast fusion. J Bacteriol. 1986 Mar;165(3):994–1001. doi: 10.1128/jb.165.3.994-1001.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman C. J., Foster T. J. Posttranscriptional regulation of the inducible nonenzymatic chloramphenicol resistance determinant of IncP plasmid R26. J Bacteriol. 1985 Jan;161(1):147–152. doi: 10.1128/jb.161.1.147-152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall E. J., Ambulos N. P., Jr, Lovett P. S. Drug-free induction of a chloramphenicol acetyltransferase gene in Bacillus subtilis by stalling ribosomes in a regulatory leader. J Bacteriol. 1987 Sep;169(9):4235–4241. doi: 10.1128/jb.169.9.4235-4241.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall E. J., Lovett P. S. Chloramphenicol induces translation of the mRNA for a chloramphenicol-resistance gene in Bacillus subtilis. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3939–3943. doi: 10.1073/pnas.83.11.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Gu Z., Harrod R., Rogers E. J., Lovett P. S. Anti-peptidyl transferase leader peptides of attenuation-regulated chloramphenicol-resistance genes. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5612–5616. doi: 10.1073/pnas.91.12.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Lovett P. S. Perturbing highly conserved spatial relationships in the regulatory domain that controls inducible cat translation. Mol Microbiol. 1992 Oct;6(19):2769–2776. doi: 10.1111/j.1365-2958.1992.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Gu Z., Rogers E. J., Lovett P. S. Peptidyl transferase inhibition by the nascent leader peptide of an inducible cat gene. J Bacteriol. 1993 Sep;175(17):5309–5313. doi: 10.1128/jb.175.17.5309-5313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod R., Gu Z., Lovett P. S. Analysis of the secondary structure that negatively regulates inducible cat translation by use of chemical probing and mutagenesis. Gene. 1994 Mar 11;140(1):79–83. doi: 10.1016/0378-1119(94)90734-x. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B., Fahnestock S. R., Nomura M. Alteration of 23 S ribosomal RNA and erythromycin-induced resistance to lincomycin and spiramycin in Staphylococcus aureus. J Mol Biol. 1973 Feb 15;74(1):67–72. doi: 10.1016/0022-2836(73)90355-0. [DOI] [PubMed] [Google Scholar]
- Lovett P. S. Translational attenuation as the regulator of inducible cat genes. J Bacteriol. 1990 Jan;172(1):1–6. doi: 10.1128/jb.172.1.1-6.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Chloramphenicol, erythromycin, carbomycin and vernamycin B protect overlapping sites in the peptidyl transferase region of 23S ribosomal RNA. Biochimie. 1987 Aug;69(8):879–884. doi: 10.1016/0300-9084(87)90215-x. [DOI] [PubMed] [Google Scholar]
- Monod M., Denoya C., Dubnau D. Sequence and properties of pIM13, a macrolide-lincosamide-streptogramin B resistance plasmid from Bacillus subtilis. J Bacteriol. 1986 Jul;167(1):138–147. doi: 10.1128/jb.167.1.138-147.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monro R. E., Marcker K. A. Ribosome-catalysed reaction of puromycin with a formylmethionine-containing oligonucleotide. J Mol Biol. 1967 Apr 28;25(2):347–350. doi: 10.1016/0022-2836(67)90146-5. [DOI] [PubMed] [Google Scholar]
- Monro R. E., Vazquez D. Ribosome-catalysed peptidyl transfer: effects of some inhibitors of protein synthesis. J Mol Biol. 1967 Aug 28;28(1):161–165. doi: 10.1016/s0022-2836(67)80085-8. [DOI] [PubMed] [Google Scholar]
- Mulbry W. W., Ambulos N. P., Jr, Lovett P. S. Bacillus subtilis mutant allele sup-3 causes lysine insertion at ochre codons: use of sup-3 in studies of translational attenuation. J Bacteriol. 1989 Oct;171(10):5322–5324. doi: 10.1128/jb.171.10.5322-5324.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwlandt D. T., Haas E. S., Daniels C. J. The RNA component of RNase P from the archaebacterium Haloferax volcanii. J Biol Chem. 1991 Mar 25;266(9):5689–5695. [PubMed] [Google Scholar]
- Rogers E. J., Ambulos N. P., Jr, Lovett P. S. Complementarity of Bacillus subtilis 16S rRNA with sites of antibiotic-dependent ribosome stalling in cat and erm leaders. J Bacteriol. 1990 Nov;172(11):6282–6290. doi: 10.1128/jb.172.11.6282-6290.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers E. J., Kim U. J., Ambulos N. P., Jr, Lovett P. S. Four codons in the cat-86 leader define a chloramphenicol-sensitive ribosome stall sequence. J Bacteriol. 1990 Jan;172(1):110–115. doi: 10.1128/jb.172.1.110-115.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers E. J., Lovett P. S. The cis-effect of a nascent peptide on its translating ribosome: influence of the cat-86 leader pentapeptide on translation termination at leader codon 6. Mol Microbiol. 1994 Apr;12(2):181–186. doi: 10.1111/j.1365-2958.1994.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brenner D. G., LeGrice S. F., Skinner S. E., Hawkins A. R. Chloramphenicol acetyltransferase gene of staphylococcal plasmid pC221. Nucleotide sequence analysis and expression studies. FEBS Lett. 1985 Jan 1;179(1):101–106. doi: 10.1016/0014-5793(85)80200-3. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Skinner R., Cundliffe E., Schmidt F. J. Site of action of a ribosomal RNA methylase responsible for resistance to erythromycin and other antibiotics. J Biol Chem. 1983 Oct 25;258(20):12702–12706. [PubMed] [Google Scholar]
- Stokes H. W., Hall R. M. Sequence analysis of the inducible chloramphenicol resistance determinant in the Tn1696 integron suggests regulation by translational attenuation. Plasmid. 1991 Jul;26(1):10–19. doi: 10.1016/0147-619x(91)90032-r. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]