Abstract
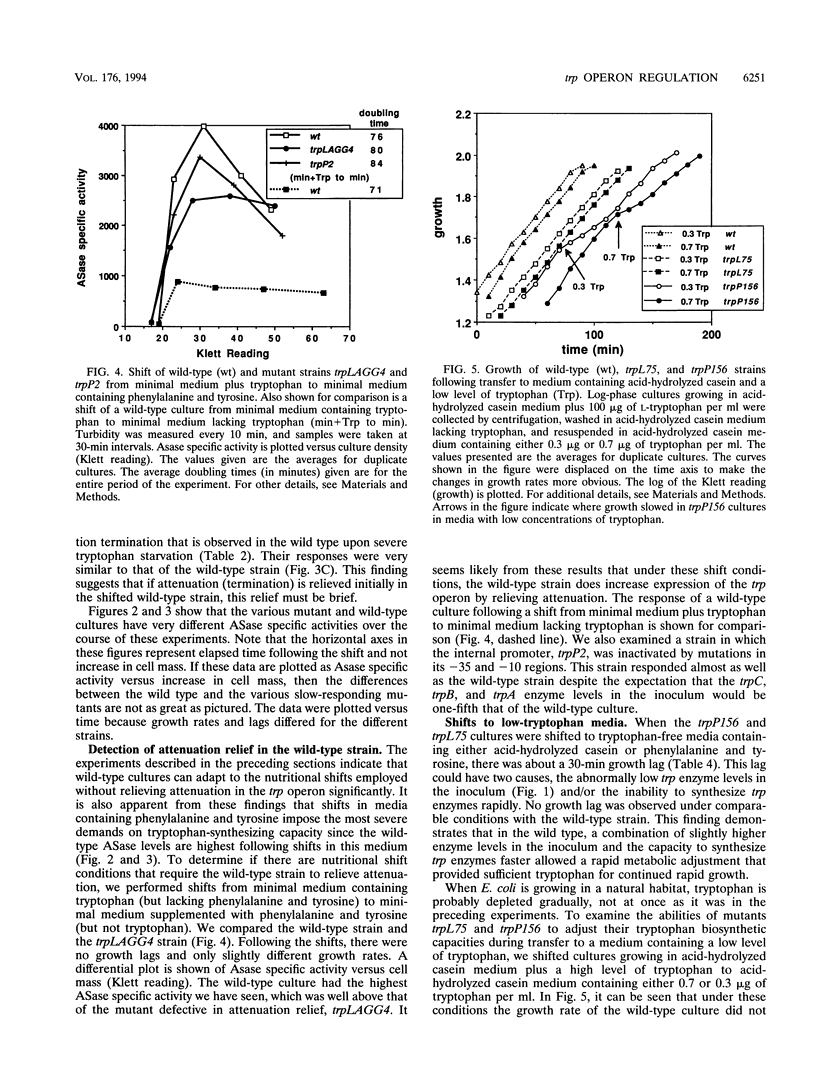
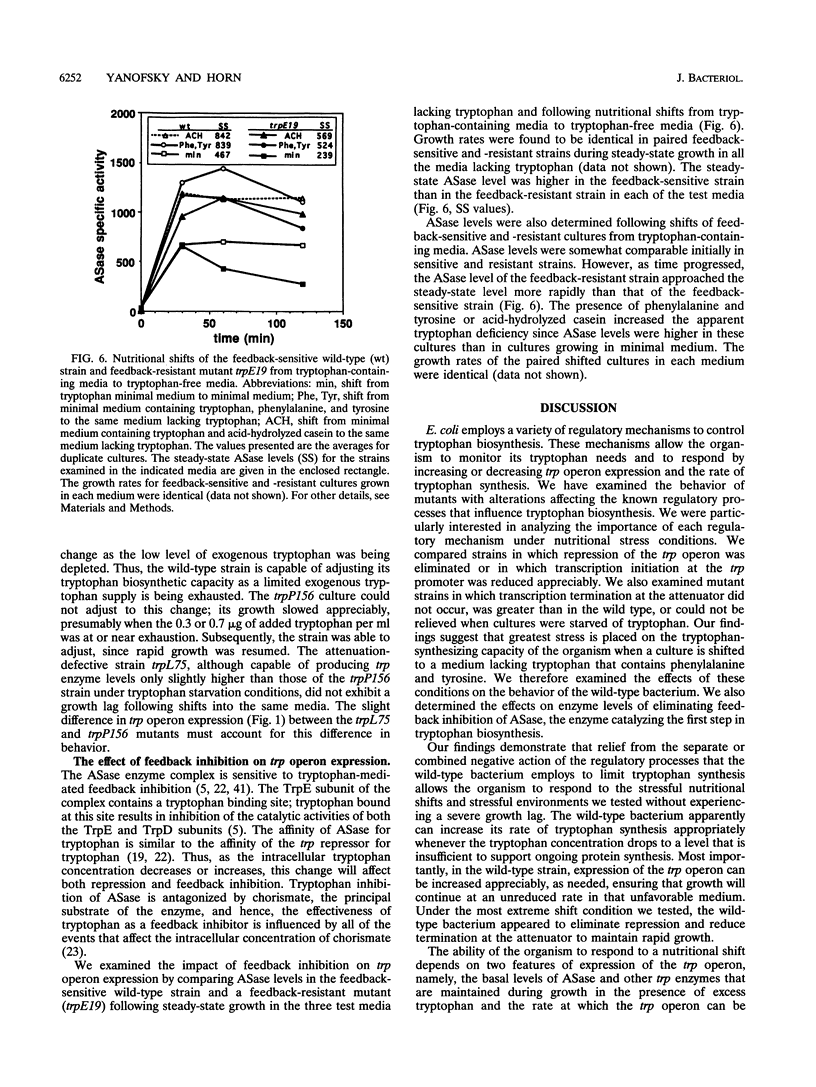
Physiological studies were performed under nutritional stress and nonstress conditions to assess the relative importance of the various regulatory mechanisms that Escherichia coli can use to alter its rate of tryptophan synthesis. Mutants were examined in which the trp repressor was inactive, transcription termination at the trp attenuator was altered, transcription initiation at the trp promoter was reduced, or feedback inhibition of anthranilate synthase was abolished. Strains were examined in media with and without tryptophan, phenylalanine and tyrosine, or acid-hydrolyzed casein and following shifts from one medium to another. Growth rates and anthranilate synthase levels were measured. In media lacking tryptophan, each of the mutants showed relief of repression and/or attenuation and maintained a near-normal growth rate. Following a shift from a medium containing tryptophan to a tryptophan-free medium containing phenylalanine and tyrosine or acid-hydrolyzed casein, mutants with abnormally low trp enzyme levels exhibited an appreciable growth lag before resuming growth. The wild-type strain displayed termination relief only under one extreme shift condition, upon transfer from a minimal medium containing tryptophan to minimal medium with only phenylalanine and tyrosine. A promoter down-mutant had difficulty adjusting to a shift from high tryptophan to low tryptophan levels in a medium containing acid-hydrolyzed casein. In all media tested, anthranilate synthase levels were lower in a feedback-resistant mutant than in the wild type. These studies demonstrate the capacity of E. coli to adjust its rate of tryptophan synthesis to maintain rapid growth following a shift to stressful nutritional conditions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURROUS S. E., DEMOSS R. D. STUDIES ON TRYPTOPHAN PERMEASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 6;73:623–637. doi: 10.1016/0006-3002(63)90332-9. [DOI] [PubMed] [Google Scholar]
- Bertrand K., Squires C., Yanofsky C. Transcription termination in vivo in the leader region of the tryptophan operon of Escherichia coli. J Mol Biol. 1976 May 15;103(2):319–337. doi: 10.1016/0022-2836(76)90315-6. [DOI] [PubMed] [Google Scholar]
- Bliss R. D., Painter P. R., Marr A. G. Role of feedback inhibition in stabilizing the classical operon. J Theor Biol. 1982 Jul 21;97(2):177–193. doi: 10.1016/0022-5193(82)90098-4. [DOI] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri M. G., Bauerle R. Subunit communication in the anthranilate synthase complex from Salmonella typhimurium. Science. 1991 Jun 28;252(5014):1845–1848. doi: 10.1126/science.2063197. [DOI] [PubMed] [Google Scholar]
- Das A., Yanofsky C. A ribosome binding site sequence is necessary for efficient expression of the distal gene of a translationally-coupled gene pair. Nucleic Acids Res. 1984 Jun 11;12(11):4757–4768. doi: 10.1093/nar/12.11.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick P., Yanofsky C. tRNA(Trp) translation of leader peptide codon 12 and other factors that regulate expression of the tryptophanase operon. J Bacteriol. 1990 Jun;172(6):3100–3107. doi: 10.1128/jb.172.6.3100-3107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Miguel A. G., Gunsalus G. L. Intracellular Trp repressor levels in Escherichia coli. J Bacteriol. 1986 Jul;167(1):272–278. doi: 10.1128/jb.167.1.272-278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatwole V. M., Somerville R. L. The tryptophan-specific permease gene, mtr, is differentially regulated by the tryptophan and tyrosine repressors in Escherichia coli K-12. J Bacteriol. 1991 Jun;173(11):3601–3604. doi: 10.1128/jb.173.11.3601-3604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz H., Platt T. Identification of trp-p2, an internal promoter in the tryptophan operon of Escherichia coli. J Mol Biol. 1982 Apr 5;156(2):257–267. doi: 10.1016/0022-2836(82)90327-8. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Thr region between the operator and first structural gene of the tryptophan operon of Escherichia coli may have a regulatory function. J Mol Biol. 1973 May 5;76(1):89–101. doi: 10.1016/0022-2836(73)90082-x. [DOI] [PubMed] [Google Scholar]
- Kelley R. L., Yanofsky C. Trp aporepressor production is controlled by autogenous regulation and inefficient translation. Proc Natl Acad Sci U S A. 1982 May;79(10):3120–3124. doi: 10.1073/pnas.79.10.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landick R., Yanofsky C., Choo K., Phung L. Replacement of the Escherichia coli trp operon attenuation control codons alters operon expression. J Mol Biol. 1990 Nov 5;216(1):25–37. doi: 10.1016/S0022-2836(05)80058-0. [DOI] [PubMed] [Google Scholar]
- Largen M., Belser W. The apparent conservation of the internal low efficiency promoter of the tryptophan operons of several species of Enterobacteriaceae. Genetics. 1973 Sep;75(1):19–22. doi: 10.1093/genetics/75.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Messenger RNA synthesis during amino acid starvation in Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):269–288. doi: 10.1016/0022-2836(68)90267-2. [DOI] [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Tryptophan messenger translation in Escherichia coli. J Mol Biol. 1970 Jul 28;51(2):435–447. doi: 10.1016/0022-2836(70)90153-1. [DOI] [PubMed] [Google Scholar]
- Marmorstein R. Q., Sigler P. B. Stereochemical effects of L-tryptophan and its analogues on trp repressor's affinity for operator-DNA. J Biol Chem. 1989 Jun 5;264(16):9149–9154. [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Kuhn J. C., Somerville R. L. Feedback regulation in the anthranilate aggregate from wild type and mutant strains of Escherichia coli. J Biol Chem. 1973 Feb 10;248(3):901–914. [PubMed] [Google Scholar]
- Roesser J. R., Nakamura Y., Yanofsky C. Regulation of basal level expression of the tryptophan operon of Escherichia coli. J Biol Chem. 1989 Jul 25;264(21):12284–12288. [PubMed] [Google Scholar]
- Roesser J. R., Yanofsky C. The effects of leader peptide sequence and length on attenuation control of the trp operon of E.coli. Nucleic Acids Res. 1991 Feb 25;19(4):795–800. doi: 10.1093/nar/19.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Yanofsky C. Metabolic regulation of the tryptophan operon of Escherichia coli: repressor-independent regulation of transcription initiation frequency. J Mol Biol. 1972 Aug 14;69(1):103–118. doi: 10.1016/0022-2836(72)90026-5. [DOI] [PubMed] [Google Scholar]
- Sarsero J. P., Wookey P. J., Gollnick P., Yanofsky C., Pittard A. J. A new family of integral membrane proteins involved in transport of aromatic amino acids in Escherichia coli. J Bacteriol. 1991 May;173(10):3231–3234. doi: 10.1128/jb.173.10.3231-3234.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsero J. P., Wookey P. J., Pittard A. J. Regulation of expression of the Escherichia coli K-12 mtr gene by TyrR protein and Trp repressor. J Bacteriol. 1991 Jul;173(13):4133–4143. doi: 10.1128/jb.173.13.4133-4143.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli D., McKenney K., Sobieski D. A., Rosenberg M. Translational coupling at an intercistronic boundary of the Escherichia coli galactose operon. Cell. 1982 Oct;30(3):865–871. doi: 10.1016/0092-8674(82)90291-4. [DOI] [PubMed] [Google Scholar]
- Stauffer G. V., Zurawski G., Yanofsky C. Single base-pair alterations in the Escherichia coli trp operon leader region that relieve transcription termination at the trp attenuator. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4833–4837. doi: 10.1073/pnas.75.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., Yanofsky C. Evidence for transcription antitermination control of tryptophanase operon expression in Escherichia coli K-12. J Bacteriol. 1985 Nov;164(2):731–740. doi: 10.1128/jb.164.2.731-740.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroynowski I., van Cleemput M., Yanofsky C. Superattenuation in the tryptophan operon of Serratia marcescens. Nature. 1982 Jul 1;298(5869):38–41. doi: 10.1038/298038a0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yanofsky C., Horn V., Gollnick P. Physiological studies of tryptophan transport and tryptophanase operon induction in Escherichia coli. J Bacteriol. 1991 Oct;173(19):6009–6017. doi: 10.1128/jb.173.19.6009-6017.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Horn V. Rifampin resistance mutations that alter the efficiency of transcription termination at the tryptophan operon attenuator. J Bacteriol. 1981 Mar;145(3):1334–1341. doi: 10.1128/jb.145.3.1334-1341.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]
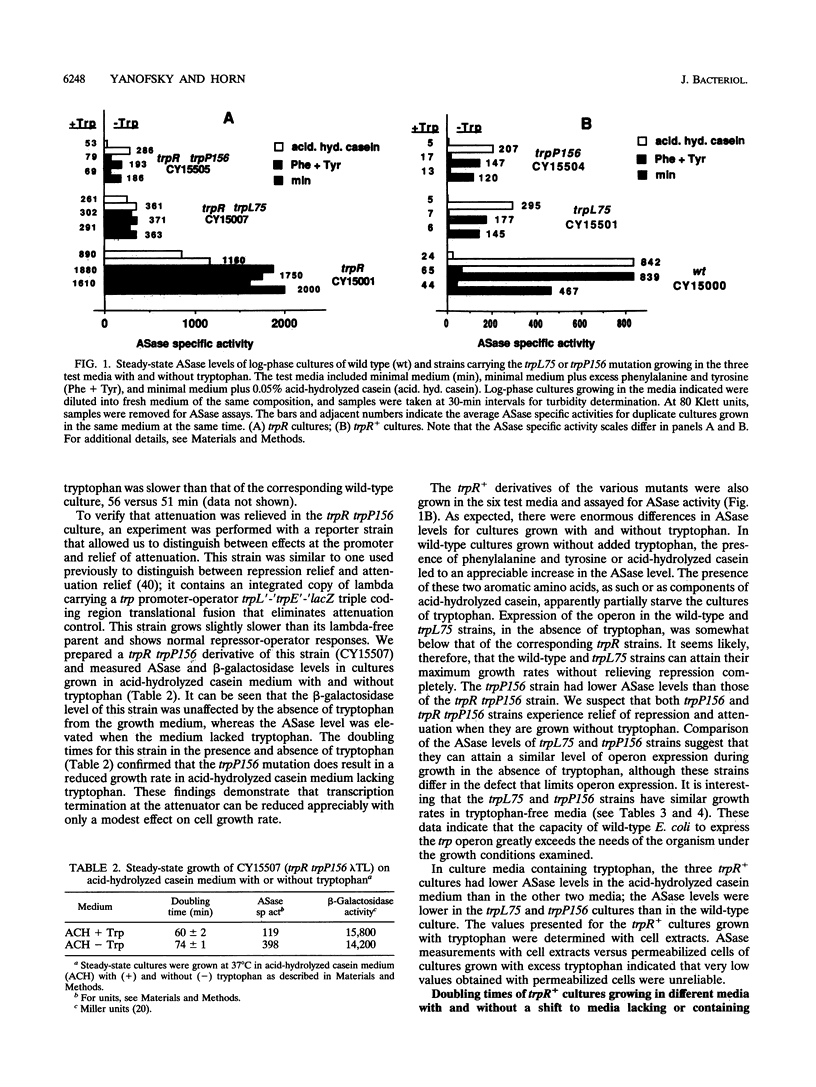
- Yanofsky C., Kelley R. L., Horn V. Repression is relieved before attenuation in the trp operon of Escherichia coli as tryptophan starvation becomes increasingly severe. J Bacteriol. 1984 Jun;158(3):1018–1024. doi: 10.1128/jb.158.3.1018-1024.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Elseviers D., Stauffer G. V., Yanofsky C. Translational control of transcription termination at the attenuator of the Escherichia coli tryptophan operon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5988–5992. doi: 10.1073/pnas.75.12.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Yanofsky C. Escherichia coli tryptophan operon leader mutations, which relieve transcription termination, are cis-dominant to trp leader mutations, which increase transcription termination. J Mol Biol. 1980 Sep 5;142(1):123–129. doi: 10.1016/0022-2836(80)90210-7. [DOI] [PubMed] [Google Scholar]