Abstract
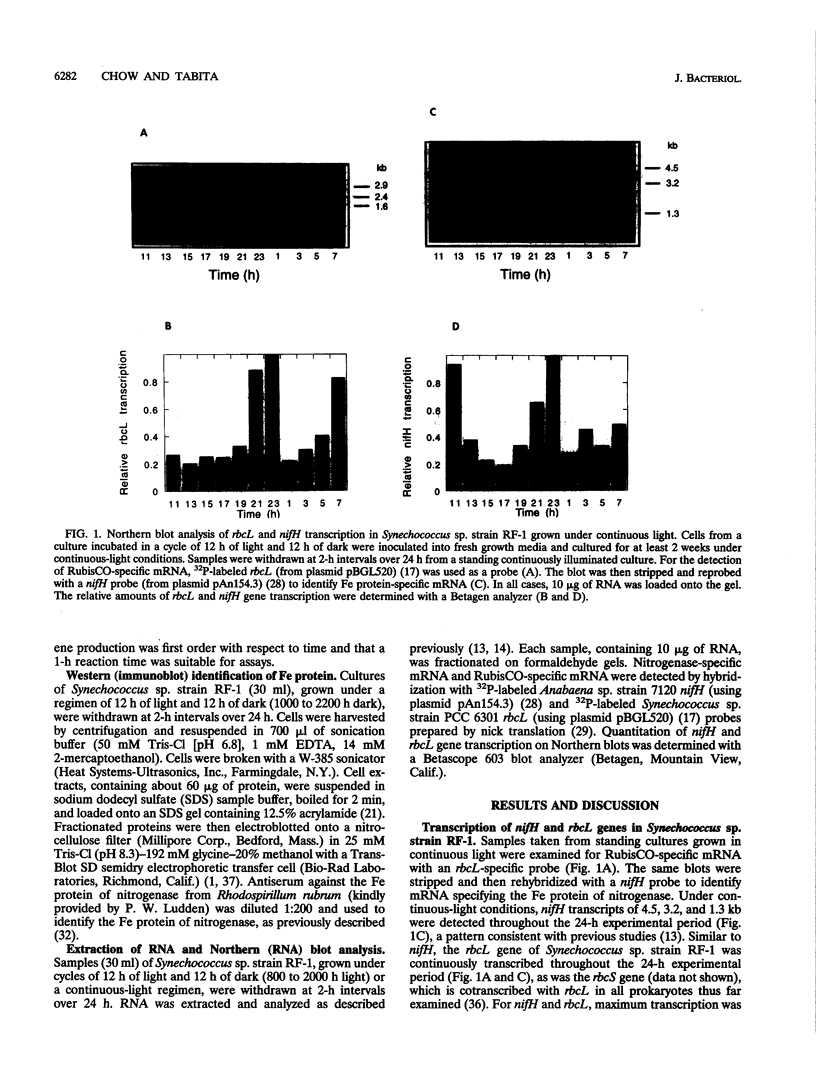
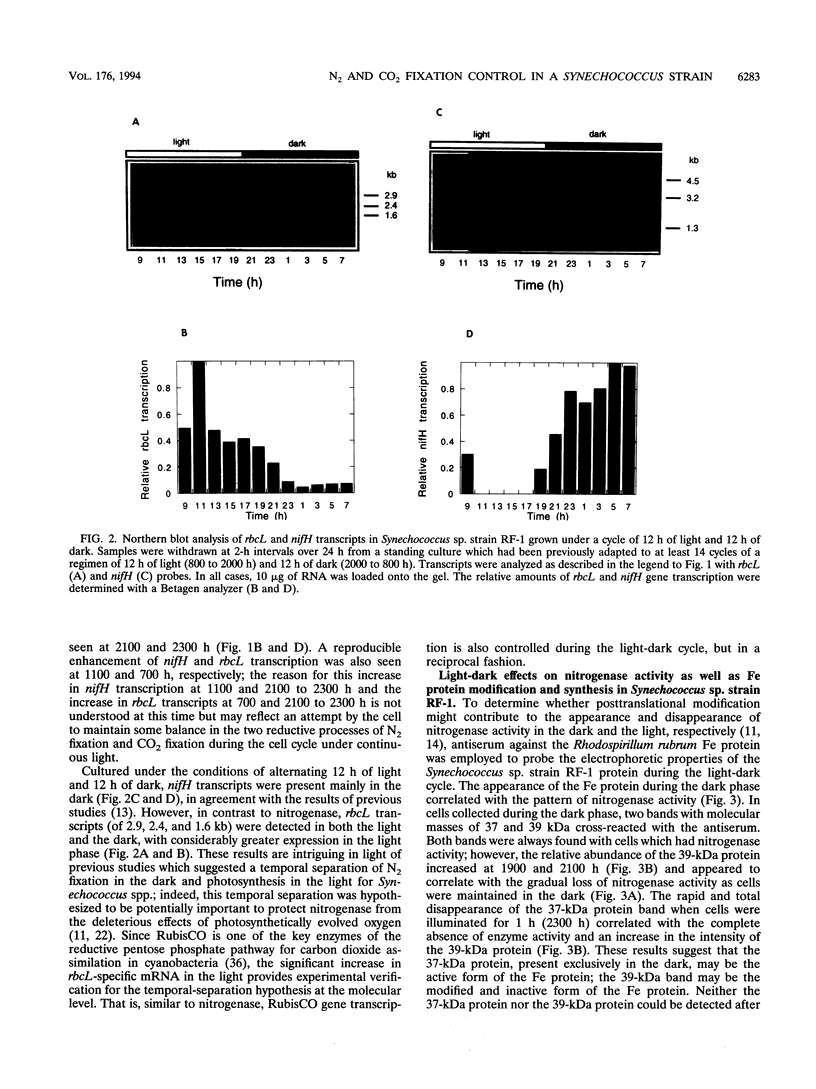
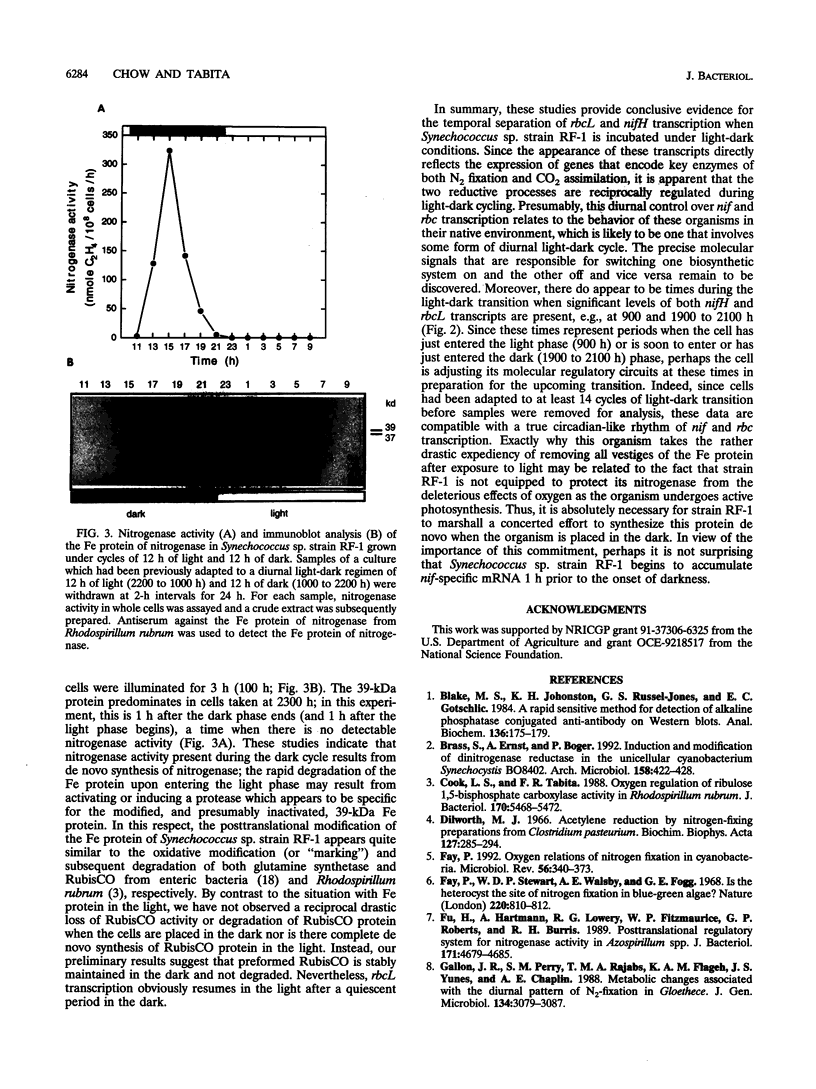
Synechococcus sp. strain RF-1 exhibits a circadian rhythm of N2 fixation when cells are grown under a light-dark cycle, with nitrogenase activity observed only during the dark period. This dark-dependent activity correlated with nif gene transcription in strain RF-1. By using antibodies against dinitrogenase reductase (the Fe protein of the nitrogenase complex), it was found that there was a distinct shift in the mobility of this protein on sodium dodecyl sulfate gels during the light-dark cycle. The Fe protein was present only when cells were incubated in the dark. Upon illumination, there was a conversion of all Fe protein to a modified form, after which it rapidly disappeared from extracts. These studies indicated that all nitrogenase activity present during the dark cycle resulted from de novo synthesis of nitrogenase. Upon entering the light phase, cells appeared to quickly degrade the modified form of Fe protein, perhaps as a result of activating or inducing a protease. By contrast, transcription of the rbcL gene, which encodes the catalytic subunit of the key enzyme of CO2 fixation (a light-dependent process), was enhanced in the light.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Cook L. S., Tabita F. R. Oxygen regulation of ribulose 1,5-bisphosphate carboxylase activity in Rhodospirillum rubrum. J Bacteriol. 1988 Dec;170(12):5468–5472. doi: 10.1128/jb.170.12.5468-5472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. J. Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim Biophys Acta. 1966 Oct 31;127(2):285–294. doi: 10.1016/0304-4165(66)90383-7. [DOI] [PubMed] [Google Scholar]
- Fay P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev. 1992 Jun;56(2):340–373. doi: 10.1128/mr.56.2.340-373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P., Stewart W. D., Walsby A. E., Fogg G. E. Is the heterocyst the site of nitrogen fixation in blue-green algae? Nature. 1968 Nov 23;220(5169):810–812. doi: 10.1038/220810b0. [DOI] [PubMed] [Google Scholar]
- Fu H. A., Hartmann A., Lowery R. G., Fitzmaurice W. P., Roberts G. P., Burris R. H. Posttranslational regulatory system for nitrogenase activity in Azospirillum spp. J Bacteriol. 1989 Sep;171(9):4679–4685. doi: 10.1128/jb.171.9.4679-4685.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotto J. W., Yoch D. C. Regulation of nitrogenase activity by covalent modification in Chromatium vinosum. Arch Microbiol. 1985 Feb;141(1):40–43. doi: 10.1007/BF00446737. [DOI] [PubMed] [Google Scholar]
- Huang T. C., Tu J., Chow T. J., Chen T. H. Circadian Rhythm of the Prokaryote Synechococcus sp. RF-1. Plant Physiol. 1990 Feb;92(2):531–533. doi: 10.1104/pp.92.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Tabita F. R. Purification of recombinant ribulose-1,5-bisphosphate carboxylase/oxygenase large subunits suitable for reconstitution and assembly of active L8S8 enzyme. Biochemistry. 1990 Oct 9;29(40):9352–9357. doi: 10.1021/bi00492a007. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Oliver C. N., Fulks R. M., Stadtman E. R. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery R. G., Ludden P. W. Purification and properties of dinitrogenase reductase ADP-ribosyltransferase from the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1988 Nov 15;263(32):16714–16719. [PubMed] [Google Scholar]
- Lowery R. G., Saari L. L., Ludden P. W. Reversible regulation of the nitrogenase iron protein from Rhodospirillum rubrum by ADP-ribosylation in vitro. J Bacteriol. 1986 May;166(2):513–518. doi: 10.1128/jb.166.2.513-518.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Pope M. R., Murrell S. A., Ludden P. W. Covalent modification of the iron protein of nitrogenase from Rhodospirillum rubrum by adenosine diphosphoribosylation of a specific arginine residue. Proc Natl Acad Sci U S A. 1985 May;82(10):3173–3177. doi: 10.1073/pnas.82.10.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K. J., Haskell J. B., Sherman D. M., Sherman L. A. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J Bacteriol. 1993 Mar;175(5):1284–1292. doi: 10.1128/jb.175.5.1284-1292.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saari L. L., Pope M. R., Murrell S. A., Ludden P. W. Studies on the activating enzyme for iron protein of nitrogenase from Rhodospirillum rubrum. J Biol Chem. 1986 Apr 15;261(11):4973–4977. [PubMed] [Google Scholar]
- Saari L. L., Triplett E. W., Ludden P. W. Purification and properties of the activating enzyme for iron protein of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1984 Dec 25;259(24):15502–15508. [PubMed] [Google Scholar]
- Smith R. L., Van Baalen C., Tabita F. R. Alteration of the Fe protein of nitrogenase by oxygen in the cyanobacterium Anabaena sp. strain CA. J Bacteriol. 1987 Jun;169(6):2537–2542. doi: 10.1128/jb.169.6.2537-2542.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]