Abstract
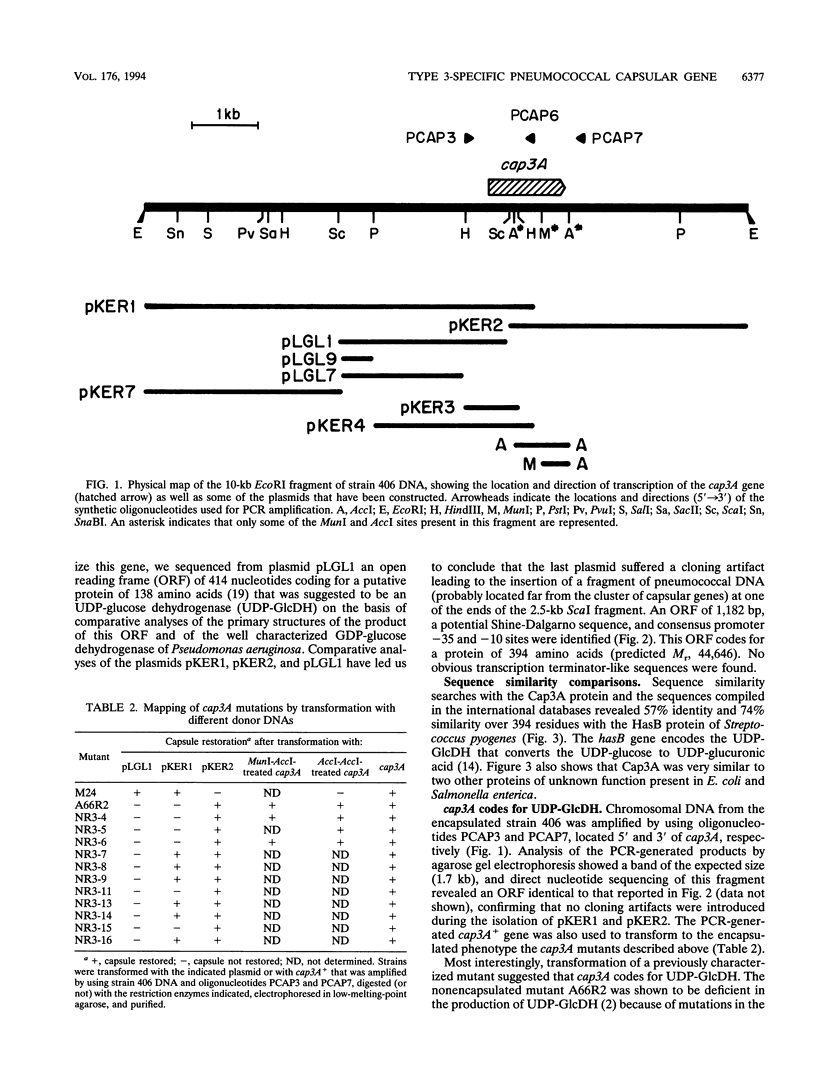
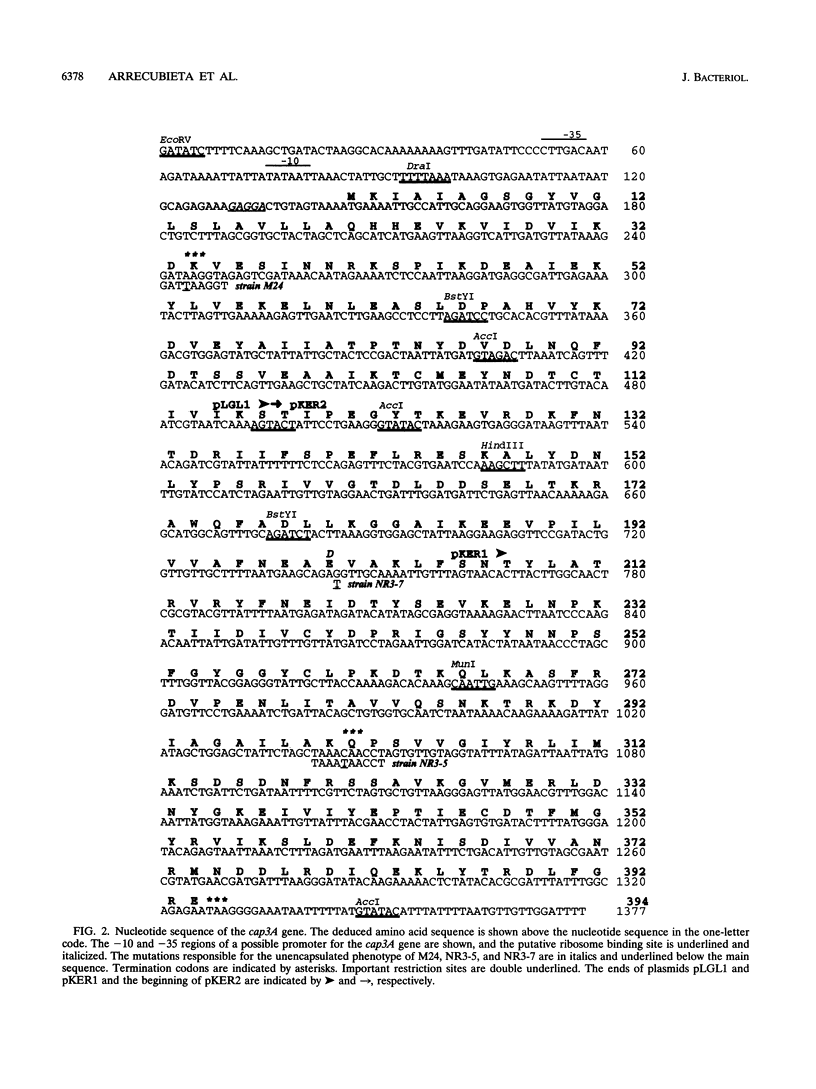
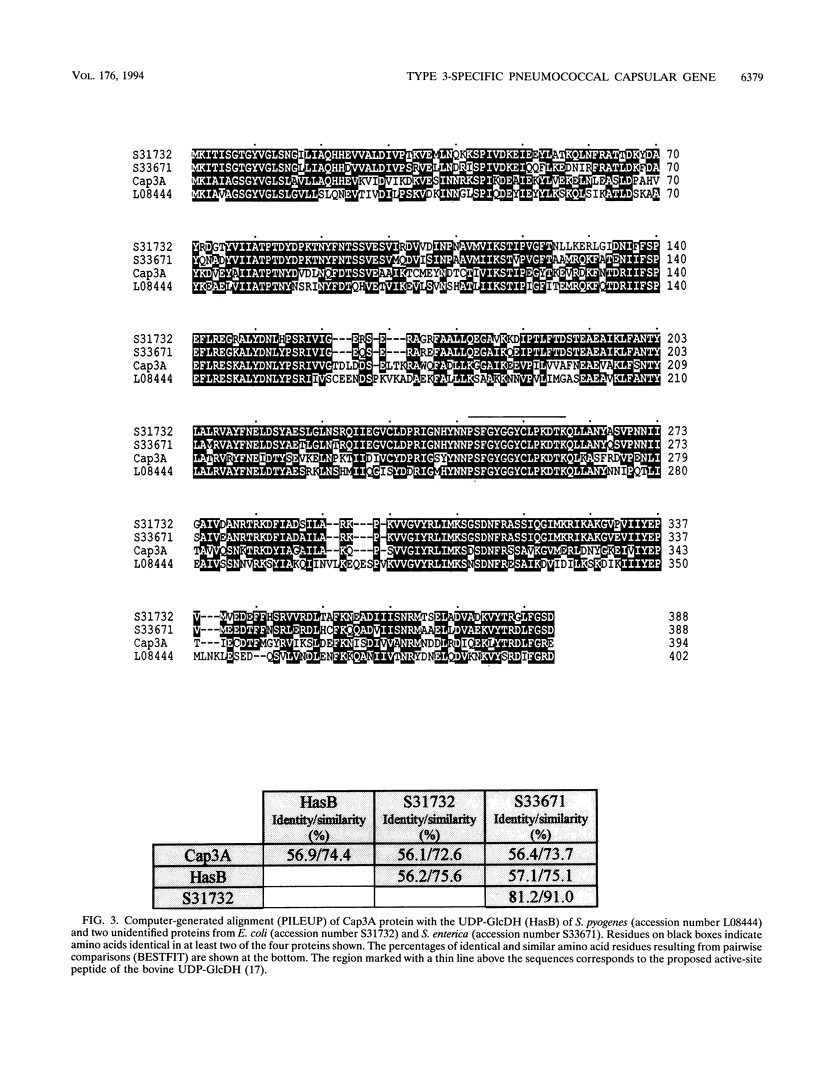
The complete nucleotide sequence of the cap3A gene of Streptococcus pneumoniae, which is directly responsible for the transformation of some unencapsulated, serotype 3 mutants to the encapsulated phenotype, has been determined. This gene encodes a protein of 394 amino acids with a predicted M(r) of 44,646. Twelve independent cap3A mutations have been mapped by genetic transformation, and three of them have been sequenced. Sequence comparisons revealed that cap3A was very similar (74.4%) to the hasB gene of Streptococcus pyogenes, which encodes a UDP-glucose dehydrogenase (UDP-GlcDH) that catalyzes the conversion of UDP-glucose to UDP-glucuronic acid, the donor substances in the pneumococcal type 3 capsular polysaccharide. Furthermore, a PCR-generated cap3A+ gene restored encapsulation in our cap3A mutants as well as in a mutant previously characterized as deficient in UDP-GlcDH (R. Austrian, H. P. Bernheimer, E.E.B. Smith, and G.T. Mills, J. Exp. Med. 110:585-602, 1959). These results support the conclusion that cap3A codes for UDP-GlcDH. We have also identified a region upstream of cap3A that should contain common genes necessary for the production of capsule of any type. Pulsed-field gel electrophoresis and Southern blotting showed that the capsular genes specific for serotype 3 are located near the genes encoding PBP 2X and PBP 1A in the S. pneumoniae chromosome, whereas copies of the common genes (or part of them) appear to be present in different locations in the genome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R., BERNHEIMER H. P., SMITH E. E., MILLS G. T. Simultaneous production of two capsular polysaccharides by pneumococcus. II. The genetic and biochemical bases of binary capsulation. J Exp Med. 1959 Oct 1;110:585–602. doi: 10.1084/jem.110.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrian R., Boettger C., Dole M., Fairly L., Freid M. Streptococcus pneumoniae type 16A, a hitherto undescribed pneumococcal type. J Clin Microbiol. 1985 Jul;22(1):127–128. doi: 10.1128/jcm.22.1.127-128.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrian R. Pneumococcus: the first one hundred years. Rev Infect Dis. 1981 Mar-Apr;3(2):183–189. doi: 10.1093/clinids/3.2.183. [DOI] [PubMed] [Google Scholar]
- Barany F., Tomasz A. Genetic transformation of Streptococcus pneumoniae by heterologous plasmid deoxyribonucleic acid. J Bacteriol. 1980 Nov;144(2):698–709. doi: 10.1128/jb.144.2.698-709.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin D. A., Stevenson G., Brown P. K., Haase A., Reeves P. R. Repeat unit polysaccharides of bacteria: a model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain length. Mol Microbiol. 1993 Mar;7(5):725–734. doi: 10.1111/j.1365-2958.1993.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Bernheimer H. P. Lysogenic pneumococci and their bacteriophages. J Bacteriol. 1979 May;138(2):618–624. doi: 10.1128/jb.138.2.618-624.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H. P., Wermundsen I. E., Austrian R. Mutation in pneumococcus type 3 affecting multiple cistrons concerned with the synthesis of capsular polysaccharide. J Bacteriol. 1968 Oct;96(4):1099–1102. doi: 10.1128/jb.96.4.1099-1102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H. P., Wermundsen I. E., Austrian R. Qualitative differences in the behavior of pneumoncoccal deoxyribonucleic acids transforming to the same capsular type. J Bacteriol. 1967 Jan;93(1):320–333. doi: 10.1128/jb.93.1.320-333.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H. P., Wermundsen I. E. Homology in capsular transformation reactions in Pneumococcus. Mol Gen Genet. 1972;116(1):68–83. doi: 10.1007/BF00334261. [DOI] [PubMed] [Google Scholar]
- Broome C. V., Breiman R. F. Pneumococcal vaccine--past, present, and future. N Engl J Med. 1991 Nov 21;325(21):1506–1508. doi: 10.1056/NEJM199111213252109. [DOI] [PubMed] [Google Scholar]
- DeAngelis P. L., Papaconstantinou J., Weigel P. H. Isolation of a Streptococcus pyogenes gene locus that directs hyaluronan biosynthesis in acapsular mutants and in heterologous bacteria. J Biol Chem. 1993 Jul 15;268(20):14568–14571. [PubMed] [Google Scholar]
- Dillard J. P., Yother J. Genetic and molecular characterization of capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 3. Mol Microbiol. 1994 Jun;12(6):959–972. doi: 10.1111/j.1365-2958.1994.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Dougherty B. A., van de Rijn I. Molecular characterization of hasB from an operon required for hyaluronic acid synthesis in group A streptococci. Demonstration of UDP-glucose dehydrogenase activity. J Biol Chem. 1993 Apr 5;268(10):7118–7124. [PubMed] [Google Scholar]
- Drake C. R., Boulnois G. J., Roberts I. S. The Escherichia coli serA-linked capsule locus and its flanking sequences are polymorphic, genetic evidence for the existence of more than two groups of capsule gene clusters. J Gen Microbiol. 1993 Aug;139(8):1707–1714. doi: 10.1099/00221287-139-8-1707. [DOI] [PubMed] [Google Scholar]
- Fenoll A., Martín Bourgon C., Muñz R., Vicioso D., Casal J. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing systemic infections in Spain, 1979-1989. Rev Infect Dis. 1991 Jan-Feb;13(1):56–60. doi: 10.1093/clinids/13.1.56. [DOI] [PubMed] [Google Scholar]
- Franzen B., Carrubba C., Feingold D. S., Ashcom J., Franzen J. S. Amino acid sequence of the tryptic peptide containing the catalytic-site thiol group of bovine liver uridine diphosphate glucose dehydrogenase. Biochem J. 1981 Dec 1;199(3):599–602. doi: 10.1042/bj1990599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch M., Weisgerber C., Meyer T. F. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1669–1673. doi: 10.1073/pnas.86.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García E., García P., López R. Cloning and sequencing of a gene involved in the synthesis of the capsular polysaccharide of Streptococcus pneumoniae type 3. Mol Gen Genet. 1993 May;239(1-2):188–195. doi: 10.1007/BF00281617. [DOI] [PubMed] [Google Scholar]
- Gasc A. M., Kauc L., Barraillé P., Sicard M., Goodgal S. Gene localization, size, and physical map of the chromosome of Streptococcus pneumoniae. J Bacteriol. 1991 Nov;173(22):7361–7367. doi: 10.1128/jb.173.22.7361-7367.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt S., Birkholz C., Zähringer U., Robertson B. D., van Putten J., Ebeling O., Frosch M. Contribution of genes from the capsule gene complex (cps) to lipooligosaccharide biosynthesis and serum resistance in Neisseria meningitidis. Mol Microbiol. 1994 Mar;11(5):885–896. doi: 10.1111/j.1365-2958.1994.tb00367.x. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr Pathogenesis of pneumococcal pneumonia. Rev Infect Dis. 1991 May-Jun;13 (Suppl 6):S509–S517. doi: 10.1093/clinids/13.supplement_6.s509. [DOI] [PubMed] [Google Scholar]
- Klugman K. P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990 Apr;3(2):171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J. S., Zamze S., Loynds B., Moxon E. R. Common organization of chromosomal loci for production of different capsular polysaccharides in Haemophilus influenzae. J Bacteriol. 1989 Jun;171(6):3343–3347. doi: 10.1128/jb.171.6.3343-3347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre J. C., Faucon G., Sicard A. M., Gasc A. M. DNA fingerprinting of Streptococcus pneumoniae strains by pulsed-field gel electrophoresis. J Clin Microbiol. 1993 Oct;31(10):2724–2728. doi: 10.1128/jcm.31.10.2724-2728.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López R., Sánchez-Puelles J. M., García E., García J. L., Ronda C., García P. Isolation, characterization and physiological properties of an autolytic-deficient mutant of Streptococcus pneumoniae. Mol Gen Genet. 1986 Aug;204(2):237–242. doi: 10.1007/BF00425504. [DOI] [PubMed] [Google Scholar]
- Nielsen S. V., Henrichsen J. Capsular types of Streptococcus pneumoniae isolated from blood and CSF during 1982-1987. Clin Infect Dis. 1992 Nov;15(5):794–798. doi: 10.1093/clind/15.5.794. [DOI] [PubMed] [Google Scholar]
- Paton J. C., Andrew P. W., Boulnois G. J., Mitchell T. J. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu Rev Microbiol. 1993;47:89–115. doi: 10.1146/annurev.mi.47.100193.000513. [DOI] [PubMed] [Google Scholar]
- Rhee D. K., Morrison D. A. Genetic transformation in Streptococcus pneumoniae: molecular cloning and characterization of recP, a gene required for genetic recombination. J Bacteriol. 1988 Feb;170(2):630–637. doi: 10.1128/jb.170.2.630-637.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I. S., Mountford R., Hodge R., Jann K. B., Boulnois G. J. Common organization of gene clusters for production of different capsular polysaccharides (K antigens) in Escherichia coli. J Bacteriol. 1988 Mar;170(3):1305–1310. doi: 10.1128/jb.170.3.1305-1310.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C. E., Heggen L. M., Haft R. F., Wessels M. R. Identification of cpsD, a gene essential for type III capsule expression in group B streptococci. Mol Microbiol. 1993 May;8(5):843–855. doi: 10.1111/j.1365-2958.1993.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J. G., Lamy F., Frazier R., Feingold D. S. UDP-glucose dehydrogenase from Escherichia coli. Purification and subunit structure. Biochim Biophys Acta. 1976 Dec 22;453(2):418–425. doi: 10.1016/0005-2795(76)90137-9. [DOI] [PubMed] [Google Scholar]
- Shapiro E. D., Austrian R. Serotypes responsible for invasive Streptococcus pneumoniae infections among children in Connecticut. J Infect Dis. 1994 Jan;169(1):212–214. doi: 10.1093/infdis/169.1.212. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga R. K., Terpstra P., Hol W. G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986 Jan 5;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]