Abstract
Thrombin cleaves its G-protein-linked seven-transmembrane domain receptor, thereby releasing a 41-aa peptide and generating a new amino terminus that acts as a tethered ligand for the receptor. Peptides corresponding to the new amino terminal end of the proteolyzed seven-transmembrane domain thrombin receptor [TR42–55, SFLLRNPNDKYEPF, also known as TRAP (thrombin receptor-activating peptide)], previously have been demonstrated to activate the receptor. In this study, we demonstrate that the 41-aa cleaved peptide, TR1–41 (MGPRRLLLVAACFSLCGPLLSARTRARRPESKATNATLDPR) is a strong platelet agonist. TR1–41 induces platelet aggregation. In whole-blood flow cytometric studies, TR1–41 was shown to be more potent than TR42–55 and almost as potent as thrombin, as determined by the degree of increase in: (i) platelet surface expression of P-selectin (reflecting α granule secretion); (ii) exposure of the fibrinogen binding site on the glycoprotein (GP) IIb-IIIa complex; and (iii) fibrinogen binding to the activated GPIIb-IIIa complex. As determined by experiments with inhibitors [prostaglandin I2, staurosporine, wortmannin, the endothelium-derived relaxing factor congener S-nitroso-N-acetylcysteine (SNAC), EDTA, EGTA, and genestein], and with Bernard-Soulier or Glanzmann’s platelets, we demonstrated that TR1–41-induced platelet activation is: (i) inhibited by cyclic AMP; (ii) mediated by protein kinase C, phosphatidyl inositol-3-kinase, myosin light chain kinase, and intracellular protein tyrosine kinases; (iii) dependent on extracellular calcium; and (iv) independent of the GPIb-IX and GPIIb-IIIa complexes. TR1–41-induced platelet activation was synergistic with TR42–55. In summary, the cleaved peptide of the seven-transmembrane domain TR (TR1–41) is a strong platelet agonist.
Keywords: flow cytometry, platelet activation, P-selectin, glycoprotein IIb-IIIa
Thrombin is one of the most physiologically important platelet agonists (1–3). Moreover, thrombin is an essential component in the hemostatic, proliferative, and inflammatory responses to injury (4). The recent identification of the seven-transmembrane domain thrombin receptor (TR) revealed a proteolytic mechanism of activation (5). Thrombin binds to the hirudin-like domain of the seven-transmembrane domain TR via its anion binding exosite and then cleaves the receptor between R41-S42, thereby releasing a cleaved peptide and forming a new amino-terminal, the latter of which acts as a tethered ligand (5). The tethered ligand hypothesis has been confirmed by a number of experiments, including the use as receptor agonists of synthetic peptides that correspond to the amino acid sequence of the new amino terminal (5, 6). Although the platelet-activating effects of these synthetic peptides initially were considered to be identical to those of thrombin, recent work has suggested platelet activation by these synthetic peptides is less potent, and via a different signal transduction pathway, than platelet activation by thrombin (7–11). Therefore, an additional mechanism of action for thrombin’s effects on platelets may be present, possibly via protease-activated receptor 3 (12) and/or via glycoprotein (GP) Ib (13). The cleaved seven-transmembrane domain TR fragment PESKATNATLDPRSFLL (TR29–45) is devoid of agonist activity for the wild-type seven-transmembrane domain TR expressed in Xenopus oocytes (5). However, the effect on platelet activation of the entire 41-aa peptide released from the seven-transmembrane domain TR has not yet been examined. In this study, we demonstrate that this cleaved peptide of the seven-transmembrane domain TR (TR1–41) is a strong platelet agonist.
MATERIALS AND METHODS
TR1–41 Synthesis.
TR1–41 (MGPRRLLLVAACFSLCGPLLSARTRARRPESKATNATLDPR, ref. 5), TR1–10 (MGPRRLLLVA), TR11–21 (ACFSLCGPLL), TR21–30 (SARTRARRPE), TR31–41 (SKATNATLDPR), TR1–20 (MGPRRLLLVAACFSLCGPLL), TR21–41 (SARTRARRPESKATNATLDPR), TR29–45 (PESKATNATLDPRSFLL), TR42–55i (inactive TR42–55 resulted from reversing the first two amino acids, FSLLRNPNDKYEPF), TR44–55 (inactive LLRNPNDKYEPF), and TR1–41s (scrambled TR1–41 by randomly rearranging the amino acid sequence to LRTNASLLVPFLTARAKSSGTREAADPPRLMCLRPLARRCG) were synthesized in the core peptide facility of the University of Massachusetts Medical Center by using a Rainin Symphony 12-port automated instrument set to perform fluorenylmethoxycarbonyl chemistry with HBTU (N,N,N′,N′,-tetramethyl-O-{1H-Benzotriazol-1-yl} uronium hexafluorophosphate)-mediated coupling. The peptides then were obtained by automated cleavage from the resin by using trifluoroacetate and appropriate scavengers. The peptides were purified by using HPLC with a 25 × 100 mm DeltaPak C18 column (Waters Millipore) and linear gradient in CH3CN with UV spectrophotometric detection at 280 nm. The isolated peptides displaced appropriate molar ratios of the constituent amino acids as determined by Accutag analyses after acid hydrolysis.
Endothelium-Derived Relaxing Factor (EDRF) Congener.
The EDRF congener S-nitroso-N-acetylcysteine (SNAC) was prepared at 22°C by reacting equimolar concentrations of fresh N-acetylcysteine with NaNO2 at acidic pH, as previously described (14). SNAC was prepared within 10 min of use, kept at 4°C, and diluted as necessary into aqueous buffer immediately before addition to assay systems.
mAbs.
S12 (Centocor) is directed against P-selectin (15). P-selectin, also referred to as CD62P, GMP-140, and PADGEM protein, is a component of the α granule membrane of resting platelets that is expressed only on the platelet surface after platelet degranulation and secretion (15). PAC1 (Cell Center, University of Pennsylvania, Philadelphia, PA) is directed against the fibrinogen binding site exposed by a conformational change in the GPIIb-IIIa complex of activated platelets (16). F26 (provided by Harvey Gralnick, National Heart, Lung, and Blood Institute, Bethesda, MD) is directed against a conformational change in fibrinogen bound to the GPIIb-IIIa complex (17, 18). Y2/51 (Dako) is directed against GPIIIa (19) and was purchased conjugated to fluorescein isothiocyanate (FITC). Unlike Y2/51, antibodies PAC1 and F26 do not bind to resting platelets (16–19). Y2/51 does not interfere with PAC1 or F26 binding and therefore can be used in the same assays (data not shown). S12, PAC1, and F26 were biotinylated as previously described (20). S12, F26, and Y2/51 are IgG, whereas PAC1 is IgM.
Whole-Blood Flow Cytometry.
The method has been previously described in detail (20). There were no centrifugation, gel filtration, vortexing, or stirring steps that could artifactually activate platelets. The protocol was approved by the Committee for the Protection of Human Subjects in Research at the University of Massachusetts Medical Center. Peripheral blood was drawn form healthy volunteers who had not ingested aspirin or other antiplatelet drugs during the previous 10 days. As indicated, some samples were drawn from a now 8-year-old boy with Bernard–Soulier syndrome (21) or a now 14-year-old boy with Glanzmann’s thrombasthenia (22). The first 2 ml of drawn blood was discarded. Blood then was drawn into a sodium citrate Vacutainer (Becton Dickinson). Within 15 min of drawing, the blood was diluted 1:20 in modified Hepes-Tyrode’s buffer (137 mM NaCl/2.8 mM KCl/1 mM MgCl2/12 mM NaHCO3/0.4 mM Na2HPO4/0.35% BSA/10 mM Hepes/5.5 mM glucose), pH 7.4. The peptide glycine-l-prolyl-l-arginyl-l-proline (GPRP, Calbiochem) at a concentration of 2.5 mM was added to the samples to prevent fibrin polymerization (20). The samples were incubated for varying times at 22°C with various concentrations of either (i) the peptides TR1–41, TR1–41s, TR1–10, TR11–20, TR21–30, TR31–41, TR1–20, TR21–41, or TR42–55 [amino acid sequence SFLLRNPNDKYEPF, also known as TR activating peptide (TRAP) (Calbiochem)], TR29–45, TR42–55i, or TR44–55; (ii) purified human α-thrombin (provided by John W. Fenton II, New York Department of Health, Albany, NY); or (iii) control buffer. In some experiments 10 units/ml of hirudin (Calbiochem), a specific thrombin inhibitor (23), was added concomitantly with the addition of the synthetic peptides or thrombin. In other experiments, the chelating agents EDTA (2 mM) or EGTA (2 mM) were added 10 min before addition of the synthetic peptides or thrombin. In some experiments, various concentrations of TR1–41 were added to various concentrations of: a) TR1–10, b) TR11–20, c) TR21–30, d) TR31–41, e) TR1–20, f) TR21–41, g) TR42–55, h) TR29–45, i) TR42–55i, j) TR44–55, k) TR1–41s for 10 min at 22°C. In other experiments, either 10 μM prostaglandin (PG) I2 (Sigma) or 10 μM SNAC (an EDRF congener), known inhibitors of platelet activation (24, 25), were added concomitantly with the addition of the synthetic peptides or thrombin. Rather than the diluted whole blood used in experiments with PGI2 and other inhibitors (see below), experiments with SNAC were performed in platelet-rich plasma diluted 1:20 in modified Hepes-Tyrode’s buffer, pH 7.4 as previously described (26). In some experiments, the following agents were incubated at 22°C for 10 min before the addition of the synthetic peptides, thrombin, or combinations of the synthetic peptides: (i) 10 μM staurosporine (Sigma), an inhibitor of protein kinase C; (ii) 100 nM or 1 μM wortmannin (Sigma), an inhibitor of phosphatidyl inositol-3-kinase, also an inhibitor of myosin light chain kinase (27, 28), or (iii) 100 μM genestein (Sigma), an inhibitor of protein tyrosine kinases (29, 30).
At various time points up to 300 sec after the addition of the agonists, all samples were fixed at 22°C for 20 min with 1% formaldehyde (final concentration). After fixation, samples were diluted 10-fold in modified Tyrode’s buffer, pH 7.4. The samples then were incubated at 22°C for 20 min with a near saturating concentration of FITC-conjugated mAb Y2/51 and a saturating concentration of biotinylated mAb S12 followed by an incubation at 22°C for 20 min with 30 μg/ml of phycoerythrin-streptavidin (Jackson ImmunoResearch). In assays using biotinylated mAbs PAC1 or F26 (rather than S12), the antibody incubation was performed before fixation, as previously described (31).
As previously described (20), samples were analyzed in an EPICS Profile flow cytometer (Coulter). The flow cytometer was equipped with a 500 mW argon laser (Cyonics, San Jose, CA) operated at 15 mW with an emission wavelength of 488 nm. The fluorescence of FITC and phycoerythrin were detected by using 525-nm and 575-nm band pass filters, respectively. After identification of platelets by gating on both Y2/51-FITC positivity (i.e., GPIIIa positivity) and their characteristic light scatter, binding of the biotinylated mAb (S12, PAC1, or F26) was determined by analyzing 5,000 individual platelets for phycoerythrin fluorescence. Background binding, obtained from parallel samples run with FITC-Y2/51 and purified biotinylated mouse IgG (IgM for PAC1 assays) (Boehringer Mannheim), was subtracted from each test sample.
Platelet Aggregometry.
Platelet aggregometry was performed in a Chronolog 560ca aggregometer (Chrono-Log, Halvertown, PA). Washed platelets were prepared as previously described (32). Blood was drawn by venepuncture into a Vacutainer, as described above. The citrated blood was centrifuged (150 × g, 15 min, 22°C), and the supernatant (platelet-rich plasma, PRP) was separated. After addition to the PRP of citrate albumin wash buffer (128 mM NaCl/4.3 mM Na2H2PO4.H2O/7.5 mM Na2HPO4/4.8 mM sodium citrate/2.4 mM citric acid/11 mM glucose/0.35% BSA), pH 6.5 with 50 ng/ml PGE1, washed platelets were prepared by centrifugation. The concentration of washed platelets was adjusted to 300,000/μl in modified Hepes-Tyrode’s buffer, pH 7.4. Various concentrations of TR1–41, TR42–55, TR29–45, TR1–10, TR42–5i, TR44–55, TR1–41s, or thrombin were added to a final volume of 500 μl of washed platelets. Aggregation was recorded as an increase in light transmission.
RESULTS
TR1–41 Activates Platelets.
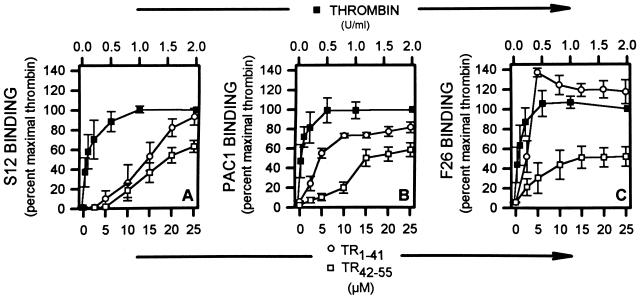
Exposure of diluted whole blood to TR1–41 resulted in a concentration-dependent increase in the surface expression of various activation-dependent antigens, as determined by whole-blood flow cytometry (Fig. 1). TR1–41 resulted in increased platelet surface expression of P-selectin (reflecting α granule secretion) (Fig. 1A). TR1–41 also resulted in increased expression of the activated conformation of the GPIIb-IIIa complex, as reported by PAC1 binding (Fig. 1B). The TR1–41-induced increase in the platelet surface binding of mAb F26 demonstrated that TR1–41 resulted in fibrinogen binding to this activated GPIIb-IIIa complex (Fig. 1C). The maximal TR1–41-induced binding of S12, PAC1, and F26 was significantly greater than with TR42–55 and almost as great as with thrombin (Fig. 1). To assess whether thrombin is required for TR1–41-induced platelet activation, platelets were activated with TR1–41 in the presence of saturating amounts (10 units/ml) of hirudin, a specific thrombin inhibitor. Hirudin did not inhibit the ability of TR1–41 or TR42–55 to activate platelets but, as expected, did inhibit thrombin-induced platelet activation. The seven-transmembrane domain TR-related fragment TR29–45, and the control TR agonist peptides TR42–55i, and TR44–55 are devoid of activity in seven-transmembrane domain TR expressing Xenopus oocytes (5). As determined by flow cytometry, 20 μM TR29–45, 20 μM TR42–55i, 20 μM TR44–55, or 20 μM TR1–41s, a peptide with a random sequence of the amino acids of TR1–41, did not result in platelet degranulation. To assess whether the entire 41-aa sequence of TR1–41 is required for platelet activation, peptides were synthesized that correspond to the first, second, third, and fourth sets of 10 sequential amino acids and the first and second sets of 20 sequential amino acids of TR1–41. None of these peptides (TR1–10, TR11–20, TR21–30, TR31–41, TR1–20, or TR21–40) resulted in platelet activation as detected by whole-blood flow cytometry.
Figure 1.
TR1–41-, TR42–55-, and thrombin-induced platelet activation. The indicated concentrations of TR1–41-, TR42–55-, and thrombin, or control buffer were incubated (22°C, 10 min) with diluted whole blood and then fixed. The platelet surface binding of the mAbs S12 (directed against P-selectin, A), PAC1 (directed against the activated GPIIb-IIIa complex, B) and F26 (directed against fibrinogen bound to the GPIIb-IIIa complex, C) were determined by whole-blood flow cytometry. Binding is expressed as a percent of the binding with maximal thrombin (2 units/ml). Data are mean ± SEM. n = 6.
Platelet aggregation studies were performed with washed platelets in response to the addition of various concentrations of TR1–41, TR42–55, TR29–45, TR1–10, TR1–41s, TR42–55i, TR44–55i, or thrombin (Table 1). The maximum amplitude of the aggregation curve for TR1–41 was slightly less than that for TR42–55 or thrombin. Moreover, the time-to-maximum aggregation for TR1–41 was greater than that of TR42–55 or thrombin and the slope of the TR1–41 aggregation curve were less than that of TR42–55 and thrombin, demonstrating slower kinetics of TR1–41-induced platelet aggregation. TR29–45, TR42–55i, TR44–55, or TR1–41s did not result in platelet aggregation (Table 1). TR1–41 did not induce platelet aggregation in platelet-rich plasma (data not shown).
Table 1.
Platelet aggregation
| Agonist | Amplitude of light transmission, % maximum | Maximum slope, % light transmission/sec | Concentration to achieve maximum aggregation | Time delay to maximum aggregation, sec | EC50 |
|---|---|---|---|---|---|
| Thrombin | 93.8 ± 1.2 | 1.5 ± 0.1 | 0.28 ± 0.13 units/ml | 47.5 ± 10.3 | 0.09 ± 0.01 units/ml |
| TR1-41 | 68 ± 11 | 0.82 ± 0.19 | 15.3 ± 2.8 μM | 62.5 ± 16.8 | 7.25 ± 1.08 μM |
| TR42-55 | 85 ± 4.7 | 1.53 ± 0.33 | 12 ± 1.2 μM | 42.7 ± 19.3 | 13 ± 2.34 μM |
| TR29-45 | 1.5 ± 0.7 | NA | NA | NA | NA |
| TR1-10 | 1.0 ± 0.6 | NA | NA | NA | NA |
| TR1-41s | 1.0 ± 0.6 | NA | NA | NA | NA |
| TR44-55i | 1.0 ± 0.4 | NA | NA | NA | NA |
| TR42-55i | 1.0 ± 0.6 | NA | NA | NA | NA |
s, Scrambled; i, inactive; NA, not applicable. Data are mean ± SEM. n = 6.
Similar to the aggregometry studies with washed platelets, the increase in platelet surface expression of P-selectin detected by flow cytometry was more rapid in response to thrombin and TR42–55 than to TR1–41 (data not shown). After 1 min of agonist-induced platelet activation, 2 units/ml of thrombin resulted in greater than 80% of maximal degranulation, 25 μM of TR42–55 resulted in greater than 60% of maximal platelet degranulation, but 25 μM of TR1–41 resulted in 7% platelet degranulation. Thrombin and TR42–55 resulted in maximal platelet degranulation within 1.5 min, whereas TR1–41 resulted in maximal platelet degranulation after 5 min.
TR1–41-induced platelet activation also was assessed in the presence of excess amounts of TR1–10, TR11–20, TR21–30, TR31–41, TR1–20, TR21–40, TR42–55i, and TR1–41s to see whether any of these peptides competed for TR1–41 binding. TR1–10, TR11–20, TR21–30, TR31–41, TR1–20, TR21–40, TR42–55i, and TR1–41s failed to inhibit TR1–41-induced platelet degranulation. Similarly, TR1–41s did not interfere with TR42–55-induced platelet activation.
TR1–41 Synergy with TR42–55.
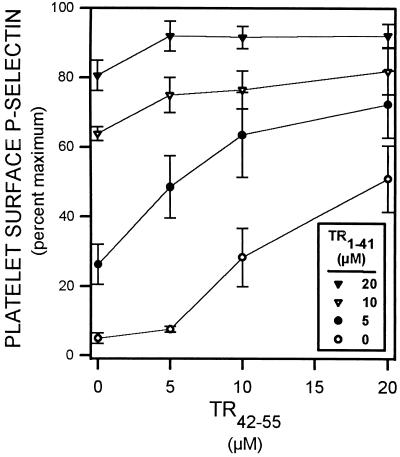
TR1–41 augmented TR42–55-induced platelet activation and TR42–55 augmented TR1–41-induced platelet activation in a concentration-dependent manner, thereby demonstrating that these two peptides act synergistically (Fig. 2).
Figure 2.
TR1–41 and TR42–55 activate platelets synergistically. Diluted whole blood was incubated (22°C, 10 min) with TR1–41 and with TR42–55 at the indicated concentrations. The platelet surface binding of the P-selectin-specific mAb S12 was determined by flow cytometry. Binding is expressed as a percent of the binding with maximal thrombin (2 units/ml). Data are mean ± SEM. n = 6.
TR1–41 Requires Extracellular Calcium to Activate Platelets.
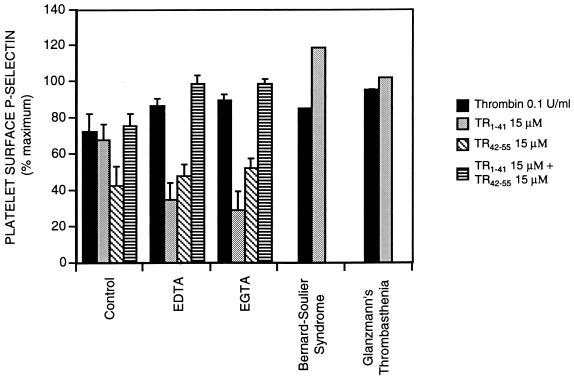
The divalent cation chelator EDTA (2 mM) and the calcium chelator EGTA (2 mM) reduced 25 μM TR1–41-induced platelet degranulation, as indicated by platelet surface P-selectin (Fig. 3). Neither EDTA nor EGTA inhibited thrombin- (2 units/ml) or TR42–55- (25 μM) induced platelet degranulation (Fig. 3). Because the platelet surface expression of P-selectin is not calcium-dependent (33), these experiments demonstrate that TR1–41 requires extracellular calcium to activate platelets.
Figure 3.
Effects of extracellular calcium and the GPIb-IX and GPIIb-IIIa complexes on TR1–41-induced platelet activation. Diluted whole blood from normal volunteers was incubated (22°C, 10 min) with TR1–41, TR42–55, concomitant TR1–41 and TR42–55, or thrombin at the indicated concentrations in the presence of buffer alone (control), 2 mM EDTA, or 2 mM EGTA. Also, diluted whole blood from a patient with either Bernard–Soulier syndrome or Glanzmann’s thrombasthenia was incubated (22°C, 10 min) with TR1–41 or thrombin at the indicated concentrations. The platelet surface binding of the P-selectin-specific mAb S12 was determined by flow cytometry. Binding is expressed as a percent of the binding with maximal thrombin (2 units/ml). Data are mean ± SEM. n = 6 except for data for Bernard–Soulier syndrome and Glanzmann’s thrombasthenia, which are means of two experiments.
TR1–41 Requires Neither GPIb-IX Nor GPIIb-IIIa Complexes to Activate Platelets.
The absence of the GPIb-IX complex (Bernard-Soulier syndrome) or the GPIIb-IIIa complex (Glanzmann’s thrombasthenia) did not reduce TR1–41-induced platelet activation (Fig. 3). In normal platelets, inhibition of the binding of fibrinogen to GPIIb-IIIa by the addition of RGD (Arg-Gly-Asp)-containing peptides (34) also did not interfere with TR1–41-induced platelet activation (data not shown).
Intracellular Signaling Pathways Involved in TR1–41-Induced Platelet Activation.
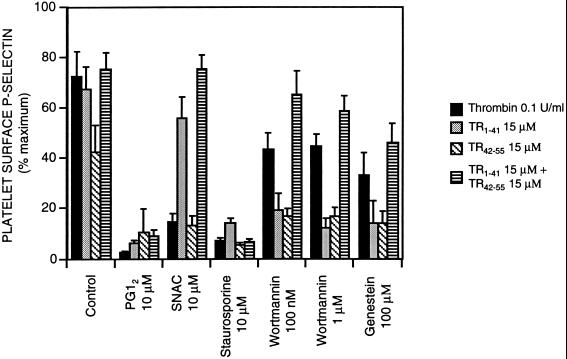
To explore the signal transduction mechanisms involved in TR1–41-induced platelet activation, we studied platelet activation by TR1–41 (and by TR42–55, concomitant TR1–41 and TR42–55, and thrombin for comparison) in the presence of agents that modify the intracellular environment (Fig. 4). PGI2, an agent that elevates intraplatelet cyclic AMP, almost completely inhibited 15 μM TR1–41-, 15 μM TR42–55-, 15 μM concomitant TR1–41-, 15 μM TR42–55-, and 0.1 units/ml of thrombin-induced platelet degranulation (as detected by S12 binding) (Fig. 4), and expression of activated GPIIb-IIIa (as detected by PAC1 and F26 binding, data not shown). The EDRF congener SNAC partially inhibited thrombin- and TR42–55-induced platelet activation, but had only minimal effect on TR1–41- and concomitant TR1–41- and TR42–55-induced platelet activation (Fig. 4). The protein kinase C inhibitor staurosporine almost completely inhibited TR1–41-, TR42–55-, concomitant TR1–41- and TR42–55-, and thrombin-induced S12 binding (Fig. 4) and PAC-1 binding (data not shown). At nanomolar concentrations, wortmannin selectively inhibits phosphatidyl inositol-3-kinase (PI3 kinase) but at higher concentrations wortmannin also inhibits myosin light chain kinase (27, 28). At a concentration of 100 nM or 1 μM, wortmannin reduced platelet surface P-selectin expression in response to TR1–41 and TR42–55 more than platelet surface P-selectin expression in response to concomitant TR1–41 and TR42–55 or thrombin (Fig. 4). Inhibition of intracellular tyrosine kinase activity by the addition of genestein (29, 30) resulted in a decrease in TR1–41-, TR42–55-, concomitant TR1–41- and TR42–55-, and thrombin-induced platelet activation (Fig. 4). Taken together, the data in Fig. 4 demonstrate that TR1–41-induced platelet activation may be modulated, at least in part, via cyclic AMP, protein kinase C, PI3 kinase, myosin light chain kinase, and intracellular protein tyrosine kinases.
Figure 4.
Intracellular signal transduction pathways involved in TR1–41-induced platelet activation. After either (i) preincubation (22°C, 30 min) with 10 μM staurosporine, 100 nM or 1 μM wortmannin, or 100 μM genestein, or (ii) coincubation with buffer only (control), 10 μM PGI2, or 10 μM the EDRF congener SNAC, diluted whole blood (or diluted platelet-rich plasma for the experiments with SNAC) were incubated (22°C, 10 min) with TR1–41, TR42–55, concomitant TR1–41 and TR42–55, or thrombin at the indicated concentrations and then fixed. The platelet surface binding of the P-selectin-specific mAb S12 was determined by whole-blood flow cytometry. Binding is expressed as a percent of the binding with maximal thrombin (2 units/ml) in normal volunteers. Data are mean ± SEM. n = 6.
DISCUSSION
In this study we report that the peptide cleaved from the seven-transmembrane domain TR (TR1–41) is a strong platelet agonist. The function of TR1–41 is not completely known, but bound to the intact native seven-transmembrane domain TR, TR1–41 inhibits receptor activation by the tethered ligand (5). Our data suggests that although TR1–41 is still bound to the seven-transmembrane domain TR it is itself sequestered and unable to activate platelets. Once cleaved by thrombin from the intact receptor, TR1–41 is more potent than TR42–55 (TR-activating peptide) and is almost as potent as thrombin, as determined by the degree of increase in: (i) the platelet surface expression of P-selectin (reflecting α granule secretion); (ii) exposure of the fibrinogen binding site on the GPIIb-IIIa complex; and (iii) fibrinogen binding to the activated GPIIb-IIIa complex. TR1–41 is a less potent aggregator of platelets than TR42–55 or thrombin. As determined by experiments with inhibitors (PGI2, staurosporine, wortmannin, SNAC, EDTA, EGTA, and genestein), and with Bernard-Soulier or Glanzmann’s platelets, we demonstrated that TR1–41-induced platelet activation is: (i) inhibited by cyclic AMP; (ii) mediated by protein kinase C, phosphatidyl inositol-3-kinase, myosin light chain kinase, and intracellular protein tyrosine kinases; (iii) dependent on extracellular calcium; and (iv) independent of the GPIb-IX and GPIIb-IIIa complexes. TR1–41-induced platelet activation was synergistic with TR42–55.
As evidenced by the lack of TR1–41-induced platelet aggregation in platelet-rich plasma, there may be a plasma inhibitor. However, paradoxically, the small amount of plasma present in the diluted whole blood used in our flow cytometric experiments allowed maximal TR1–41-induced platelet activation. Thus, the effects of plasma on TR1–41-induced platelet activation remain to be fully characterized.
Coughlin’s laboratory reported that the cleaved seven-transmembrane domain TR fragment TR29–45 was devoid of agonist activity for the wild-type seven-transmembrane domain TR expressed by direct expression cloning in Xenopus oocytes (5). In our study TR29–45 did not activate platelets as determined by platelet surface expression of P-selectin and did not result in platelet aggregation. These findings are consistent with our data that platelet activation only occurs with the entire 41-aa cleaved peptide.
The cleaved peptide of the TR has been predicted to be less than 41 amino acids in length because of a hydrophobic sequence with potential peptidase cleavage sites located at amino acids T24 and A26 (5). However, it has been recently reported by Ramachandran et al. (35) that a mAb with an epitope that binds to the first half of TR1–41 binds to intact and TR42–55-activated platelets but not to receptors that have been cleaved and activated by thrombin. Furthermore, this mAb fails to bind to the receptor after cathepsin G cleavage, but does bind to platelets activated by agonists that are unrelated to thrombin (35). Thus, while the precise amino acid length of the mature thrombin receptor on the platelet surface is unknown, available evidence (35) suggests that at least residues 19–41 are expressed on the platelet surface.
The physiologic significance of platelet activation by TR1–41 is unclear. Assuming (i) 1,000–2,000 seven-transmembrane domain TRs on the resting platelet surface (36), (ii) this number increases by 40% when platelets are activated because of the translocation of TRs from the surface connecting membrane system to the plasma membrane (37), and (iii) all of the resultant available TRs are cleaved by thrombin, the concentration of TR1–41 generated by cleavage of all available seven-transmembrane domain TRs would be, based on the present data, insufficient to activate platelets [(3 × 1011 platelets/liter of blood) × (2,100 molecules/platelet) ÷ (6.023 × 1023 molecules) = 1.05 nM]. However, at sites of thrombus formation such as vessel injury the local concentration of TR1–41 is likely to be such that TR1–41-induced platelet activation occurs. Furthermore, similar to its effects with TR42–55, TR1–41 may act synergistically with other platelet agonists such as ADP, epinephrine, and thromboxane A2 to enhance thrombus formation. Thus, TR1–41 may augment platelet activation in a growing thrombus. Such a role for TR1–41 would be consistent with the slower kinetics of TR1–41-induced platelet activation compared with thrombin- or TR42–55-induced platelet activation.
In summary, the cleaved peptide of the seven-transmembrane domain TR (TR1–41) is a strong platelet agonist. Further work will be required to define the role of TR1–41 in platelet physiology and hemostasis. Important areas for future investigation include identification of a binding site of TR1–41 on the platelet surface, other components of the intracellular signal transduction pathways, and other cells responsive to TR1–41. Identification of other cells responsive to TR1–41 will be important in light of the recent finding that seven-transmembrane domain TRs are present on the endothelial layer of normal human arteries, and in smooth muscle and intimal cells in human atheroma (38). Because the presence of the seven-transmembrane domain TR in these tissues implies the local generation of TR1–41, TR1–41 may be involved in the pathophysiology of atherosclerosis and restenosis.
Acknowledgments
We thank Drs. John W. Fenton II and Harvey Gralnick for generously providing reagents.
ABBREVIATIONS
- TR
thrombin receptor
- SNAC
S-nitroso-N-acetylcysteine
- GP
glycoprotein
- EDRF
endothelium-derived relaxing factor
- FITC
fluorescein isothiocyanate
- PG
prostaglandin
References
- 1.Hansen S R, Harker L A. Proc Natl Acad Sci USA. 1988;85:3184–3188. doi: 10.1073/pnas.85.9.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eidt J F, Allison P, Nobel S, Ashton J, Golino P, McNatt J, Buja L M, Willerson J T. J Clin Invest. 1989;84:18–27. doi: 10.1172/JCI114138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly A B, Marzec U M, Krupski W, Bass A, Cadroy Y, Hanson S R, Harker L A. Blood. 1991;77:1006–1012. [PubMed] [Google Scholar]
- 4.Coughlin S R, Vu T K H, Huang D T, Wheaton V I. J Clin Invest. 1992;89:351–355. doi: 10.1172/JCI115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vu T K, Hung D T, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 6.Vu T-K, Wheaton V I, Hung D T, Coughlin S R. Nature (London) 1991;353:674–677. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- 7.Tesfamariam B. Circ Res. 1994;74:930–936. doi: 10.1161/01.res.74.5.930. [DOI] [PubMed] [Google Scholar]
- 8.Vouret-Craviari V, Van Obbergahn-Schilling E, Scimeca J C, Van Obbergahn E, Pouyssegur J. Biochem J. 1993;289:209–214. doi: 10.1042/bj2890209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinlough-Rathbone R L, Perry D W, Packham M A. Thromb Hemostasis. 1995;73:122–125. [PubMed] [Google Scholar]
- 10.Jenkins A L, Bootman M D, Berridge M J, Stone S R. J Biol Chem. 1994;269:17104–17110. [PubMed] [Google Scholar]
- 11.Lau L F, Pumiglia K, Cote Y P, Feinstein M B. Biochem J. 1994;303:391–400. doi: 10.1042/bj3030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishihara H, Connolly A J, Zeng D, Kahn M L, Zheng Y W, Timmons C, Tram T, Coughlin S R. Nature (London) 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto N, Greco N J, Barnard M R, Tanoue K, Yamazaki H, Jamieson G A, Michelson A D. Blood. 1991;77:1740–1748. [PubMed] [Google Scholar]
- 14.Mendelsohn M E, O’Neill S, George D, Loscalzo J. J Biol Chem. 1990;265:19028–19034. [PubMed] [Google Scholar]
- 15.McEver R P. Blood Cells. 1990;16:73–80. [PubMed] [Google Scholar]
- 16.Shattil S, Hoxie J, Cunningham M, Brass L F. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- 17.Gralnick H R, Williams S B, McKeown L, Connaghan G D, Shafer B, Hansmann K, Vail M, Fenton J. J Lab Clin Med. 1991;118:604–613. [PubMed] [Google Scholar]
- 18.Gralnick H R, Williams S B, McKeown L, Shafer B, Connaghan G D, Hansmann K, Vail M, Magruder L. Br J Haematol. 1992;80:347–357. doi: 10.1111/j.1365-2141.1992.tb08144.x. [DOI] [PubMed] [Google Scholar]
- 19.Gatter K C, Cordell J L, Turley H, Heryet A, Kieffer N, Anstee D J, Mason D Y. Histopathology. 1988;13:257–267. doi: 10.1111/j.1365-2559.1988.tb02037.x. [DOI] [PubMed] [Google Scholar]
- 20.Michelson A D, Ellis P A, Barnard M R, Matic G B, Viles A F, Kestin A S. Blood. 1991;77:770–779. [PubMed] [Google Scholar]
- 21.LaRosa C A, Rohrer M J, Benoit S E, Barnard M R, Michelson A D. Blood. 1994;84:158–168. [PubMed] [Google Scholar]
- 22.Michelson A D. J Lab Clin Med. 1987;110:346–354. [PubMed] [Google Scholar]
- 23.Markwardt F. Semin Thromb Hemostasis. 1989;15:269–282. doi: 10.1055/s-2007-1002719. [DOI] [PubMed] [Google Scholar]
- 24.Michelson A D, Benoit S E, Furman M I, Breckwoldt W L, Rohrer M J, Barnard M R, Loscalzo J. Am J Physiol Heart Circ Physiol. 1996;270:H1640–H1649. doi: 10.1152/ajpheart.1996.270.5.H1640. [DOI] [PubMed] [Google Scholar]
- 25.Schini V B, Vanhoutte P M. In: Endothelium-Derived Vasoactive Factors. Loscalzo J, Schafer A I, editors. Boston: Blackwell; 1994. pp. 349–367. [Google Scholar]
- 26.Michelson A D, Benoit S E, Furman M I, Breckwoldt W L, Rohrer M J, Barnard M R, Loscalzo J. Am J Physiol Heart Circ Physiol. 1996;39:H1640–H1648. doi: 10.1152/ajpheart.1996.270.5.H1640. [DOI] [PubMed] [Google Scholar]
- 27.Kovacsovics T J, Bachelot C, Toker A, Vlahos C J, Duckworth B, Cantley L C, Hartwig J H. J Biol Chem. 1995;270:11358–11366. doi: 10.1074/jbc.270.19.11358. [DOI] [PubMed] [Google Scholar]
- 28.Thomason P A, James S R, Casey P J, Downes C P. J Biol Chemi. 1994;269:16525–16528. [PubMed] [Google Scholar]
- 29.Furman M I, Grigoryev D, Bray P F, Dise K R, Goldschmidt-Clermont P J. Circ Res. 1994;75:172–180. doi: 10.1161/01.res.75.1.172. [DOI] [PubMed] [Google Scholar]
- 30.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S-I, Itoh N, Shibuya M, Fukayami Y. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 31.Michelson A D, Barnard M R, Hechtman H B, MacGregor H, Connolly R J, Loscalzo J, Valeri C R. Proc Natl Acad Sci USA. 1996;93:11877–11882. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michelson A D, Barnard M R. Blood. 1990;76:2005–2010. [PubMed] [Google Scholar]
- 33.Hsu-Lin S, Berman C L, Furie B C, August D, Furie B. J Biol Chem. 1984;259:9121–9126. [PubMed] [Google Scholar]
- 34.Dennis M S, Henzel W J, Pitti R M, Lipari M T, Napier M A, Deisher T A, Bunting S, Lazarus R A. Proc Natl Acad Sci USA. 1990;87:2471–2475. doi: 10.1073/pnas.87.7.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramachandran R, Klufas A S, Molino M, Ahuja M, Hoxie J A, Brass L F. Thromb Hemostasis. 1997;78:1119–1124. [PubMed] [Google Scholar]
- 36.Brass L F, Vassallo R R J, Belmonte E, Ahuja M, Cichowski K, Hoxie J A. J Biol Chem. 1992;267:13795–13798. [PubMed] [Google Scholar]
- 37.Molino M, Bainton D F, Hoxie J A, Coughlin S R, Brass L F. J Biol Chem. 1997;272:6011–6017. doi: 10.1074/jbc.272.9.6011. [DOI] [PubMed] [Google Scholar]
- 38.Nelken N A, Soifer S J, O’Keefe J O, Vu T K H, Charo I F, Coughlin S R. J Clin Invest. 1992;90:1614–1621. doi: 10.1172/JCI116031. [DOI] [PMC free article] [PubMed] [Google Scholar]