Abstract
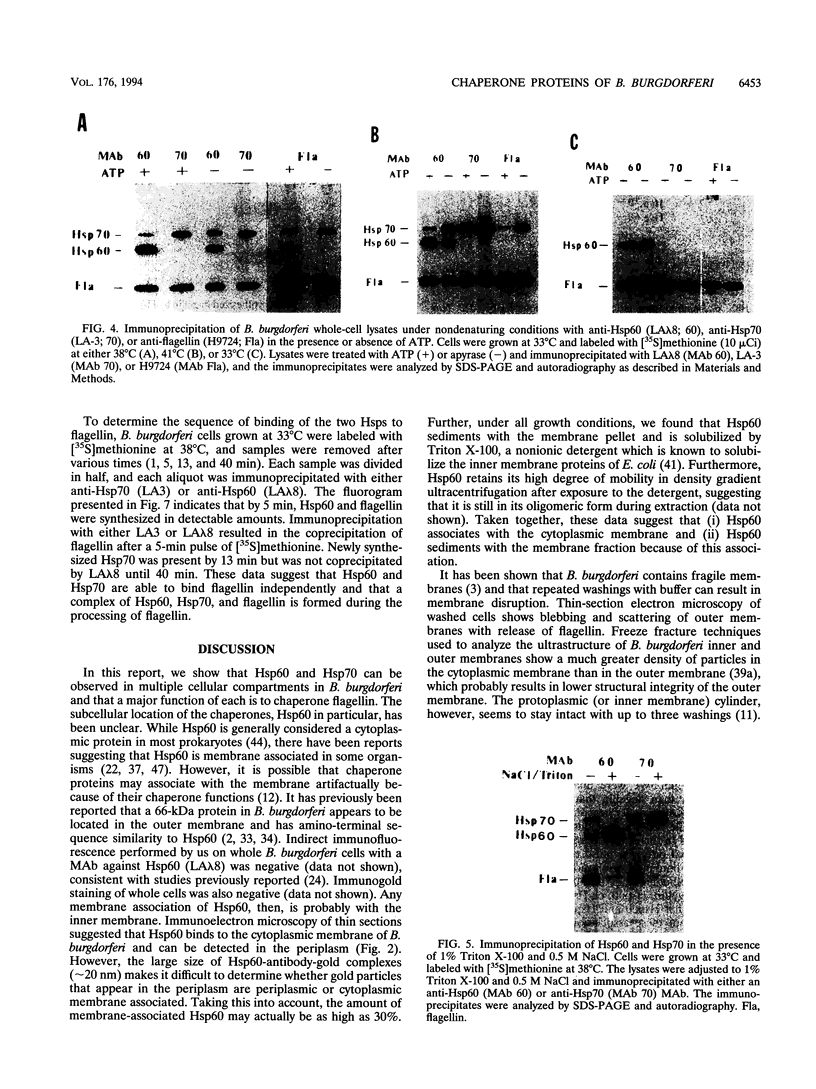
Subcellular locations and chaperone functions of Hsp60 and Hsp70 with flagellin were investigated in Borrelia burgdorferi. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot (immunoblot) analysis of fractionated cells showed Hsp60 to be present in the soluble fractions and the Triton X-100 detergent-soluble membrane fraction at growth temperatures ranging from 20 to 37 degrees C. The relative amount of Hsp60 associated with the membrane increased with growth temperature. Hsp70 was found in soluble fractions at growth temperatures between 28 and 37 degrees C, but at 20 degrees C it was also present in the Triton X-100-insoluble membrane fraction. Immunoelectron microscopy revealed that the majority of Hsp60 was localized in the cytoplasm but a detectable fraction (approximately 30%) was associated with the cell envelope. The chaperone functions of Hsp60 and Hsp70 were analyzed by immunoprecipitation of [35S]methionine-labeled cell lysates under nondenaturing conditions in the presence or absence of ATP. Hsp70 was found to bind flagellin at all temperatures tested between 33 and 41 degrees C. This association could be decreased with ATP when cells had been incubated at 41 degrees C during radioactive labeling but not at lower temperatures. Both flagellin and Hsp70 were found to associate with Hsp60, forming a complex of the three proteins. Hsp70 association with this complex could be decreased with ATP, but flagellin binding to Hsp60 was ATP independent at all temperatures studied. Both Hsp70 and flagellin were inaccessible to monoclonal antibodies against them when bound to Hsp60. These studies suggest that in B. burgdorferi, a major function of Hsp60 and Hsp70 is in the molecular processing of flagellin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Burgdorfer W., Grunwaldt E., Steere A. C. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest. 1983 Aug;72(2):504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F. Biology of Borrelia species. Microbiol Rev. 1986 Dec;50(4):381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Zylicz M., Georgopoulos C. Escherichia coli DnaK protein possesses a 5'-nucleotidase activity that is inhibited by AppppA. J Bacteriol. 1986 Nov;168(2):931–935. doi: 10.1128/jb.168.2.931-935.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulard C., Lecroisey A. Specific antisera produced by direct immunization with slices of polyacrylamide gel containing small amounts of protein. J Immunol Methods. 1982;50(2):221–226. doi: 10.1016/0022-1759(82)90228-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braig K., Simon M., Furuya F., Hainfeld J. F., Horwich A. L. A polypeptide bound by the chaperonin groEL is localized within a central cavity. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3978–3982. doi: 10.1073/pnas.90.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusca J. S., McDowall A. W., Norgard M. V., Radolf J. D. Localization of outer surface proteins A and B in both the outer membrane and intracellular compartments of Borrelia burgdorferi. J Bacteriol. 1991 Dec;173(24):8004–8008. doi: 10.1128/jb.173.24.8004-8008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B., Reilly P., McCarty J., Walker G. C. Immunogold localization of the DnaK heat shock protein in Escherichia coli cells. J Gen Microbiol. 1993 Jan;139(1):95–99. doi: 10.1099/00221287-139-1-95. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Carreiro M. M., Laux D. C., Nelson D. R. Characterization of the heat shock response and identification of heat shock protein antigens of Borrelia burgdorferi. Infect Immun. 1990 Jul;58(7):2186–2191. doi: 10.1128/iai.58.7.2186-2191.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Cohen P. S., Rossoll R., Cabelli V. J., Yang S. L., Laux D. C. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983 Apr;40(1):62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Duray P. H., Steere A. C. Clinical pathologic correlations of Lyme disease by stage. Ann N Y Acad Sci. 1988;539:65–79. doi: 10.1111/j.1749-6632.1988.tb31839.x. [DOI] [PubMed] [Google Scholar]
- Ewing C., Scorpio A., Nelson D. R., Mather T. N. Isolation of Borrelia burgdorferi from saliva of the tick vector, Ixodes scapularis. J Clin Microbiol. 1994 Mar;32(3):755–758. doi: 10.1128/jcm.32.3.755-758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Gillis T. P., Miller R. A., Young D. B., Khanolkar S. R., Buchanan T. M. Immunochemical characterization of a protein associated with Mycobacterium leprae cell wall. Infect Immun. 1985 Aug;49(2):371–377. doi: 10.1128/iai.49.2.371-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard L., Laux D. C., Jindal S., Nelson D. R. Immune recognition of human Hsp60 by Lyme disease patient sera. Microb Pathog. 1993 Apr;14(4):287–297. doi: 10.1006/mpat.1993.1028. [DOI] [PubMed] [Google Scholar]
- Hansen K., Bangsborg J. M., Fjordvang H., Pedersen N. S., Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988 Aug;56(8):2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix R. W. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979 Apr 15;129(3):375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- Holmquist L., Nelson D. R., Kjelleberg S. The DnaK homologue of the marine Vibrio sp. strain S14 binds to the unprocessed form of a carbon starvation-specific periplasmic protein. Mol Microbiol. 1994 Mar;11(5):861–868. doi: 10.1111/j.1365-2958.1994.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Höll-Neugebauer B., Rudolph R., Schmidt M., Buchner J. Reconstitution of a heat shock effect in vitro: influence of GroE on the thermal aggregation of alpha-glucosidase from yeast. Biochemistry. 1991 Dec 17;30(50):11609–11614. doi: 10.1021/bi00114a001. [DOI] [PubMed] [Google Scholar]
- Jones C. J., Macnab R. M. Flagellar assembly in Salmonella typhimurium: analysis with temperature-sensitive mutants. J Bacteriol. 1990 Mar;172(3):1327–1339. doi: 10.1128/jb.172.3.1327-1339.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Langer T., Pfeifer G., Martin J., Baumeister W., Hartl F. U. Chaperonin-mediated protein folding: GroES binds to one end of the GroEL cylinder, which accommodates the protein substrate within its central cavity. EMBO J. 1992 Dec;11(13):4757–4765. doi: 10.1002/j.1460-2075.1992.tb05581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Gorevic P. D., Jiang W., Munoz P., Dattwyler R. J. Immunologic and structural characterization of the dominant 66- to 73-kDa antigens of Borrelia burgdorferi. J Immunol. 1991 Apr 15;146(8):2776–2782. [PubMed] [Google Scholar]
- Luft B. J., Jiang W., Munoz P., Dattwyler R. J., Gorevic P. D. Biochemical and immunological characterization of the surface proteins of Borrelia burgdorferi. Infect Immun. 1989 Nov;57(11):3637–3645. doi: 10.1128/iai.57.11.3637-3645.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty J. S., Walker G. C. DnaK as a thermometer: threonine-199 is site of autophosphorylation and is critical for ATPase activity. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9513–9517. doi: 10.1073/pnas.88.21.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minota S., Cameron B., Welch W. J., Winfield J. B. Autoantibodies to the constitutive 73-kD member of the hsp70 family of heat shock proteins in systemic lupus erythematosus. J Exp Med. 1988 Oct 1;168(4):1475–1480. doi: 10.1084/jem.168.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Hsp70 accelerates the recovery of nucleolar morphology after heat shock. EMBO J. 1984 Dec 20;3(13):3095–3100. doi: 10.1002/j.1460-2075.1984.tb02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Res P. C., Schaar C. G., Breedveld F. C., van Eden W., van Embden J. D., Cohen I. R., de Vries R. R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988 Aug 27;2(8609):478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Shi W., Zhou Y., Wild J., Adler J., Gross C. A. DnaK, DnaJ, and GrpE are required for flagellum synthesis in Escherichia coli. J Bacteriol. 1992 Oct;174(19):6256–6263. doi: 10.1128/jb.174.19.6256-6263.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C. Pathogenesis of Lyme arthritis. Implications for rheumatic disease. Ann N Y Acad Sci. 1988;539:87–92. doi: 10.1111/j.1749-6632.1988.tb31841.x. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Hindersson P., de Bruyn J., Cremers F., van der Zee J., de Cock H., Tommassen J., van Eden W., van Embden J. D. Antigenic relatedness of a strongly immunogenic 65 kDA mycobacterial protein antigen with a similarly sized ubiquitous bacterial common antigen. Microb Pathog. 1988 Jan;4(1):71–83. doi: 10.1016/0882-4010(88)90049-6. [DOI] [PubMed] [Google Scholar]
- Tilly K., Georgopoulos C. Evidence that the two Escherichia coli groE morphogenetic gene products interact in vivo. J Bacteriol. 1982 Mar;149(3):1082–1088. doi: 10.1128/jb.149.3.1082-1088.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C. A heat shock operon in Coxiella burnetti produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J Bacteriol. 1988 Mar;170(3):1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]