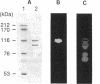
Abstract
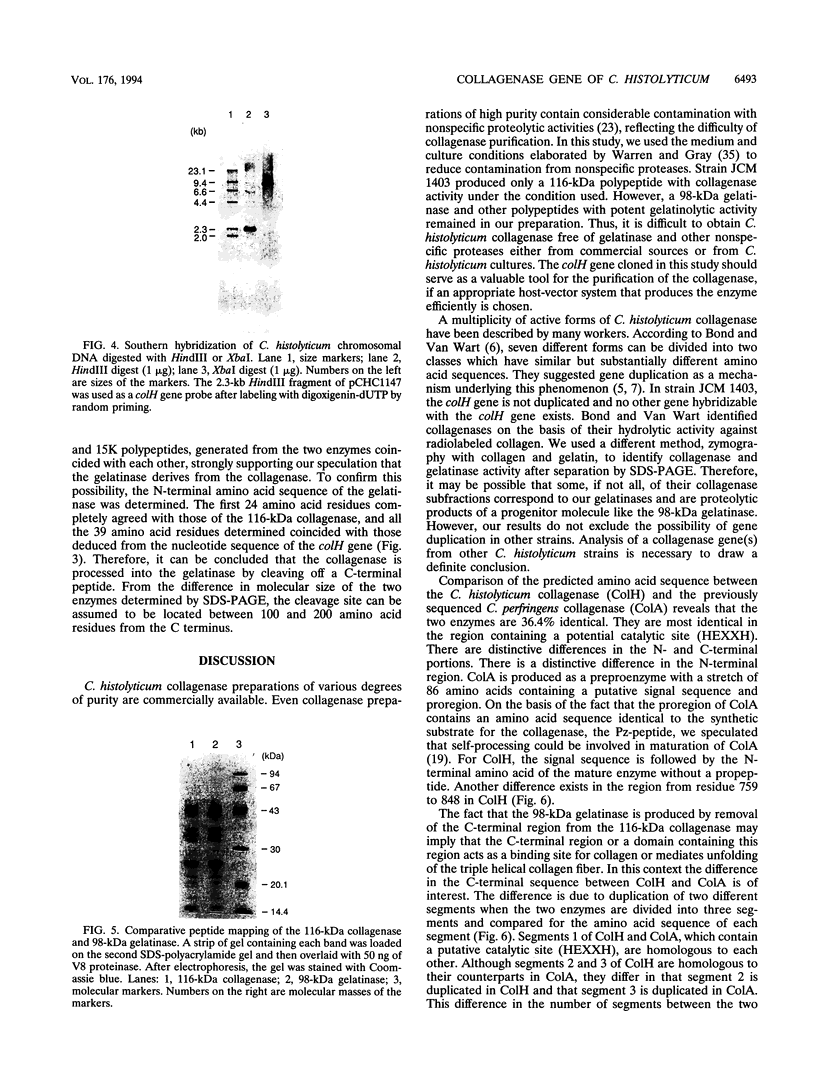
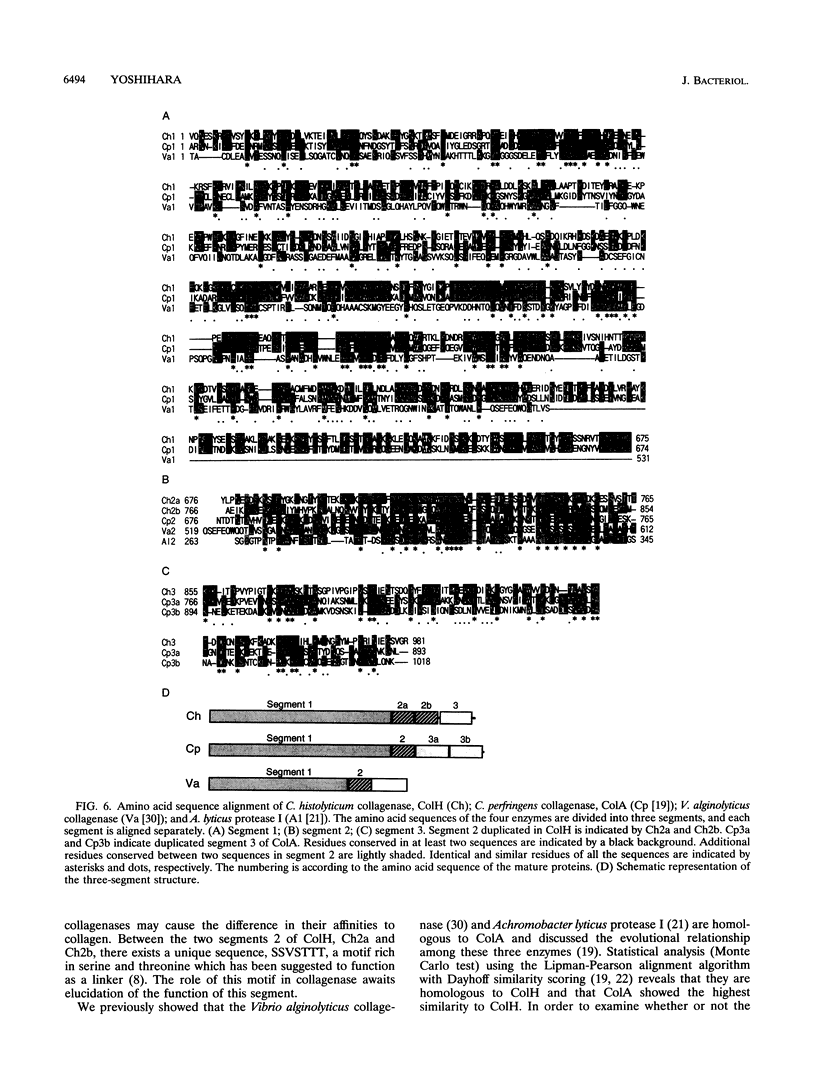
The colH gene encoding a collagenase was cloned from Clostridium histolyticum JCM 1403. Nucleotide sequencing showed a major open reading frame encoding a 116-kDa protein of 1,021 amino acid residues. The deduced amino acid sequence contains a putative signal sequence and a zinc metalloprotease consensus sequence, HEXXH. A 116-kDa collagenase and a 98-kDa gelatinase were copurified from culture supernatants of C. histolyticum. While the former degraded both native and denatured collagen, the latter degraded only denatured collagen. Peptide mapping with V8 protease showed that all peptide fragments, except a few minor ones, liberated from the two enzymes coincided with each other. Analysis of the N-terminal amino acid sequence of the two enzymes revealed that their first 24 amino acid residues were identical and coincided with those deduced from the nucleotide sequence. These results indicate that the 98-kDa gelatinase is generated from the 116-kDa collagenase by cleaving off the C-terminal region, which could be responsible for binding or increasing the accessibility of the collagenase to native collagen fibers. The role of the C-terminal region in the functional and evolutional aspects of the collagenase was further studied by comparing the amino acid sequence of the C. histolyticum collagenase with those of three homologous enzymes: the collagenases from Clostridium perfringens and Vibrio alginolyticus and Achromobacter lyticus protease I.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Taylor R. E. Detergent-activation of latent collagenase and resolution of its component molecules. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1173–1178. doi: 10.1016/s0006-291x(82)80120-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M. D., Van Wart H. E. Characterization of the individual collagenases from Clostridium histolyticum. Biochemistry. 1984 Jun 19;23(13):3085–3091. doi: 10.1021/bi00308a036. [DOI] [PubMed] [Google Scholar]
- Bond M. D., Van Wart H. E. Purification and separation of individual collagenases of Clostridium histolyticum using red dye ligand chromatography. Biochemistry. 1984 Jun 19;23(13):3077–3085. doi: 10.1021/bi00308a035. [DOI] [PubMed] [Google Scholar]
- Bond M. D., Van Wart H. E. Relationship between the individual collagenases of Clostridium histolyticum: evidence for evolution by gene duplication. Biochemistry. 1984 Jun 19;23(13):3092–3099. doi: 10.1021/bi00308a037. [DOI] [PubMed] [Google Scholar]
- Gilkes N. R., Henrissat B., Kilburn D. G., Miller R. C., Jr, Warren R. A. Domains in microbial beta-1, 4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991 Jun;55(2):303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatheway C. L. Toxigenic clostridia. Clin Microbiol Rev. 1990 Jan;3(1):66–98. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häse C. C., Finkelstein R. A. Bacterial extracellular zinc-containing metalloproteases. Microbiol Rev. 1993 Dec;57(4):823–837. doi: 10.1128/mr.57.4.823-837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Bond J. S. Families of metalloendopeptidases and their relationships. FEBS Lett. 1992 Nov 9;312(2-3):110–114. doi: 10.1016/0014-5793(92)80916-5. [DOI] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Keil B., Gilles A. M., Lecroisey A., Hurion N., Tong N. T. Specificity of collagenase from Achromobacter iophagus. FEBS Lett. 1975 Aug 15;56(2):292–296. doi: 10.1016/0014-5793(75)81112-4. [DOI] [PubMed] [Google Scholar]
- Kono T. Purification and partial characterization of collagenolytic enzymes from Clostridium histolyticum. Biochemistry. 1968 Mar;7(3):1106–1114. doi: 10.1021/bi00843a031. [DOI] [PubMed] [Google Scholar]
- Lwebuga-Mukasa J. S., Harper E., Taylor P. Collagenase enzymes from Clostridium: characterization of individual enzymes. Biochemistry. 1976 Oct 19;15(21):4736–4741. doi: 10.1021/bi00666a031. [DOI] [PubMed] [Google Scholar]
- Matsushita O., Yoshihara K., Katayama S., Minami J., Okabe A. Purification and characterization of Clostridium perfringens 120-kilodalton collagenase and nucleotide sequence of the corresponding gene. J Bacteriol. 1994 Jan;176(1):149–156. doi: 10.1128/jb.176.1.149-156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi M., Rosenbloom J. General proteolytic activity of highly purified preparations of clostridial collagenase. Connect Tissue Res. 1974;2(2):77–84. doi: 10.3109/03008207409152091. [DOI] [PubMed] [Google Scholar]
- Ohara T., Makino K., Shinagawa H., Nakata A., Norioka S., Sakiyama F. Cloning, nucleotide sequence, and expression of Achromobacter protease I gene. J Biol Chem. 1989 Dec 5;264(34):20625–20631. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassenfeld H. M. Engineering proteins for purification. Trends Biotechnol. 1990 Apr;8(4):88–93. doi: 10.1016/0167-7799(90)90145-n. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Shibano Y., Morihara K., Fukushima J., Inami S., Keil B., Gilles A. M., Kawamoto S., Okuda K. Structural gene and complete amino acid sequence of Vibrio alginolyticus collagenase. Biochem J. 1992 Feb 1;281(Pt 3):703–708. doi: 10.1042/bj2810703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart H. E., Steinbrink D. R. Complementary substrate specificities of class I and class II collagenases from Clostridium histolyticum. Biochemistry. 1985 Nov 5;24(23):6520–6526. doi: 10.1021/bi00344a032. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Giroux E. L. Haemorrhagic and inflammatory properties of collagenase from C. histolyticum. Agents Actions. 1976 Sep;6(5):627–635. doi: 10.1007/BF01971582. [DOI] [PubMed] [Google Scholar]
- WARREN G. H., GRAY J. A simplified culture medium for the production of collagenase. Nature. 1961 Nov 25;192:755–756. doi: 10.1038/192755a0. [DOI] [PubMed] [Google Scholar]
- WUENSCH E., HEIDRICH H. G. ZUR QUANTITATIVEN BESTIMMUNG DER KOLLAGENASE. Hoppe Seylers Z Physiol Chem. 1963;333:149–151. doi: 10.1515/bchm2.1963.333.1.149. [DOI] [PubMed] [Google Scholar]
- Wilson M. J., Strasser M., Vogel M. M., Sinha A. A. Calcium-dependent and calcium-independent gelatinolytic proteinase activities of the rat ventral prostate and its secretion: characterization and effect of castration and testosterone treatment. Biol Reprod. 1991 May;44(5):776–785. doi: 10.1095/biolreprod44.5.776. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoshida E., Noda H. Isolation and characterization of collagenases I and II from Clostridium histolyticum. Biochim Biophys Acta. 1965 Sep 20;105(3):562–574. doi: 10.1016/s0926-6593(65)80239-9. [DOI] [PubMed] [Google Scholar]