Abstract
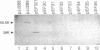
In vivo recombinants generating chimeras between the transcriptional activators VnfA and AnfA of Azotobacter vinelandii were constructed by cloning their structural genes in tandem and selecting against a conditionally lethal gene inserted between them. The parent molecules differ in their promoter specificities and in that AnfA, but not VnfA, requires the Fe protein of nitrogenase for its activity. Chimeras with fusion junctions in the N-terminal half of the central domain were found to be inactive, probably as a result of misfolding. All chimeras carrying the C-terminal domain of AnfA showed the corresponding promoter specificity, supporting the model which ascribes promoter specificity to the DNA-binding properties of the C-terminal domain. None of the chimeras showed the dependence on Fe protein typical of AnfA, including one which composed 82% of AnfA with only a short segment of VnfA at the N terminus. Deleting the N-terminal domain of AnfA gave a fully active protein which was also independent of Fe protein. This indicates that the N-terminal domain has an inhibitory effect on activity which is relieved by Fe protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L. T., Cannon F., Dean D. R. Nucleotide sequence and mutagenesis of the nifA gene from Azotobacter vinelandii. Mol Microbiol. 1988 May;2(3):315–321. doi: 10.1111/j.1365-2958.1988.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Beynon J. L., Williams M. K., Cannon F. C. Expression and functional analysis of the Rhizobium meliloti nifA gene. EMBO J. 1988 Jan;7(1):7–14. doi: 10.1002/j.1460-2075.1988.tb02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Cannon W. Mutations in the RNA polymerase recognition sequence of the Klebsiella pneumoniae nifH promoter permitting transcriptional activation in the absence of NifA binding to upstream activator sequences. Nucleic Acids Res. 1989 Apr 11;17(7):2597–2612. doi: 10.1093/nar/17.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R., Kennedy C., Kondorosi A., Krishnapillai V., Merrick M. Complementation analysis of Klebsiella pneumoniae mutants defective in nitrogen fixation. Mol Gen Genet. 1977 Nov 29;157(2):189–198. doi: 10.1007/BF00267397. [DOI] [PubMed] [Google Scholar]
- Dixon R. Tandem promoters determine regulation of the Klebsiella pneumoniae glutamine synthetase (glnA) gene. Nucleic Acids Res. 1984 Oct 25;12(20):7811–7830. doi: 10.1093/nar/12.20.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M. H., Contreras A., Mitchenall L. A. The function of isolated domains and chimaeric proteins constructed from the transcriptional activators NifA and NtrC of Klebsiella pneumoniae. Mol Microbiol. 1990 Jan;4(1):29–37. doi: 10.1111/j.1365-2958.1990.tb02012.x. [DOI] [PubMed] [Google Scholar]
- Drummond M. H., Wootton J. C. Sequence of nifL from Klebsiella pneumoniae: mode of action and relationship to two families of regulatory proteins. Mol Microbiol. 1987 Jul;1(1):37–44. doi: 10.1111/j.1365-2958.1987.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Drummond M., Whitty P., Wootton J. Sequence and domain relationships of ntrC and nifA from Klebsiella pneumoniae: homologies to other regulatory proteins. EMBO J. 1986 Feb;5(2):441–447. doi: 10.1002/j.1460-2075.1986.tb04230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler S., Drummond M. A counterselectable pACYC184-based lacZ alpha-complementing plasmid vector with novel multiple cloning sites; construction of chromosomal deletions in Klebsiella pneumoniae. Gene. 1993 Dec 22;136(1-2):253–255. doi: 10.1016/0378-1119(93)90474-h. [DOI] [PubMed] [Google Scholar]
- Gronenborn B. Overproduction of phage lambda repressor under control of the lac promotor of Escherichia coli. Mol Gen Genet. 1976 Nov 17;148(3):243–250. doi: 10.1007/BF00332898. [DOI] [PubMed] [Google Scholar]
- Jacob J., Drummond M. Construction of chimeric proteins from the sigma N-associated transcriptional activators VnfA and AnfA of Azotobacter vinelandii shows that the determinants of promoter specificity lie outside the 'recognition' helix of the HTH motif in the C-terminal domain. Mol Microbiol. 1993 Nov;10(4):813–821. doi: 10.1111/j.1365-2958.1993.tb00951.x. [DOI] [PubMed] [Google Scholar]
- Joerger R. D., Jacobson M. R., Bishop P. E. Two nifA-like genes required for expression of alternative nitrogenases by Azotobacter vinelandii. J Bacteriol. 1989 Jun;171(6):3258–3267. doi: 10.1128/jb.171.6.3258-3267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Wolfinger E. D., Bishop P. E. The gene encoding dinitrogenase reductase 2 is required for expression of the second alternative nitrogenase from Azotobacter vinelandii. J Bacteriol. 1991 Jul;173(14):4440–4446. doi: 10.1128/jb.173.14.4440-4446.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V. A common set of conserved motifs in a vast variety of putative nucleic acid-dependent ATPases including MCM proteins involved in the initiation of eukaryotic DNA replication. Nucleic Acids Res. 1993 Jun 11;21(11):2541–2547. doi: 10.1093/nar/21.11.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Morett E., Segovia L. The sigma 54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J Bacteriol. 1993 Oct;175(19):6067–6074. doi: 10.1128/jb.175.19.6067-6074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Kofoid E. C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- Ried J. L., Collmer A. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene. 1987;57(2-3):239–246. doi: 10.1016/0378-1119(87)90127-2. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Hedge P. J., te Heesen S., Edelman A., Broome-Smith J. K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41(2-3):337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Shanmugam K. T., Ausubel F., Morandi C., Goldberg R. B. Regulation of nitrogen fixation in Klebsiella pneumoniae: evidence for a role of glutamine synthetase as a regulator of nitrogenase synthesis. J Bacteriol. 1974 Nov;120(2):815–821. doi: 10.1128/jb.120.2.815-821.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H., Weissmann C. Formation of genes coding for hybrid proteins by recombination between related, cloned genes in E. coli. Nucleic Acids Res. 1983 Aug 25;11(16):5661–5669. doi: 10.1093/nar/11.16.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton J. C., Drummond M. H. The Q-linker: a class of interdomain sequences found in bacterial multidomain regulatory proteins. Protein Eng. 1989 May;2(7):535–543. doi: 10.1093/protein/2.7.535. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]