Abstract
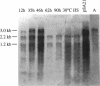
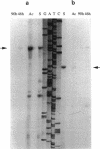
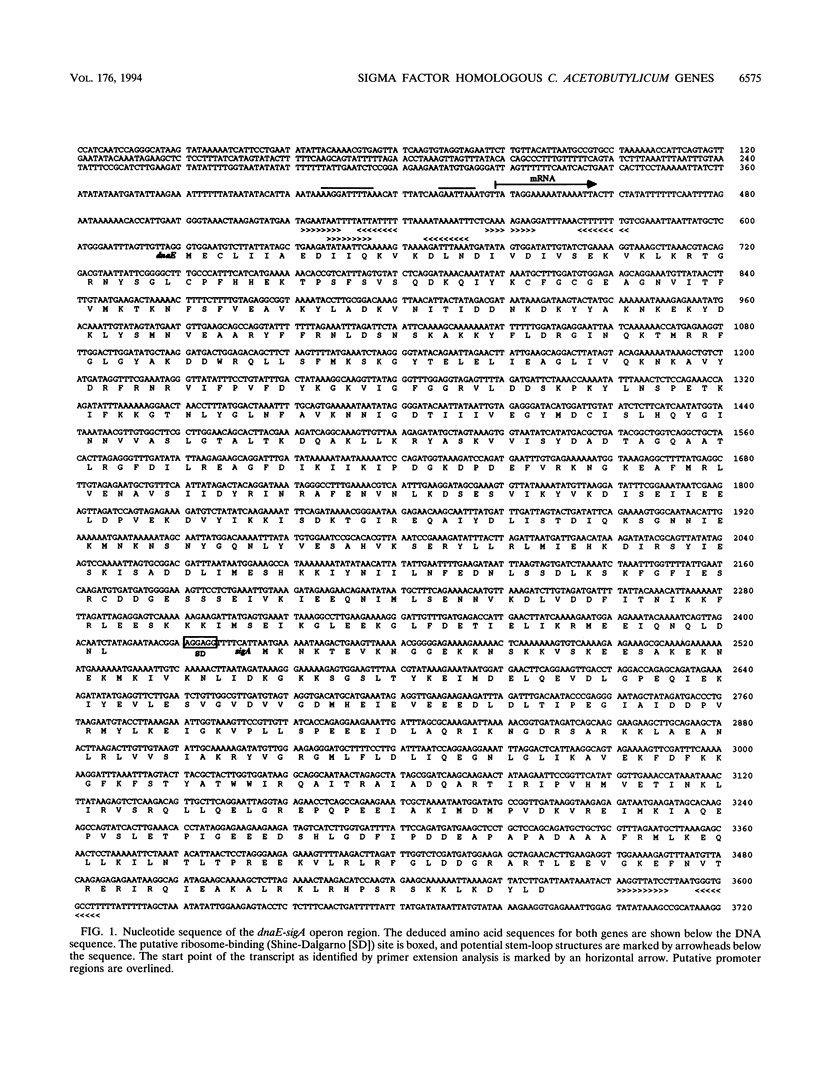
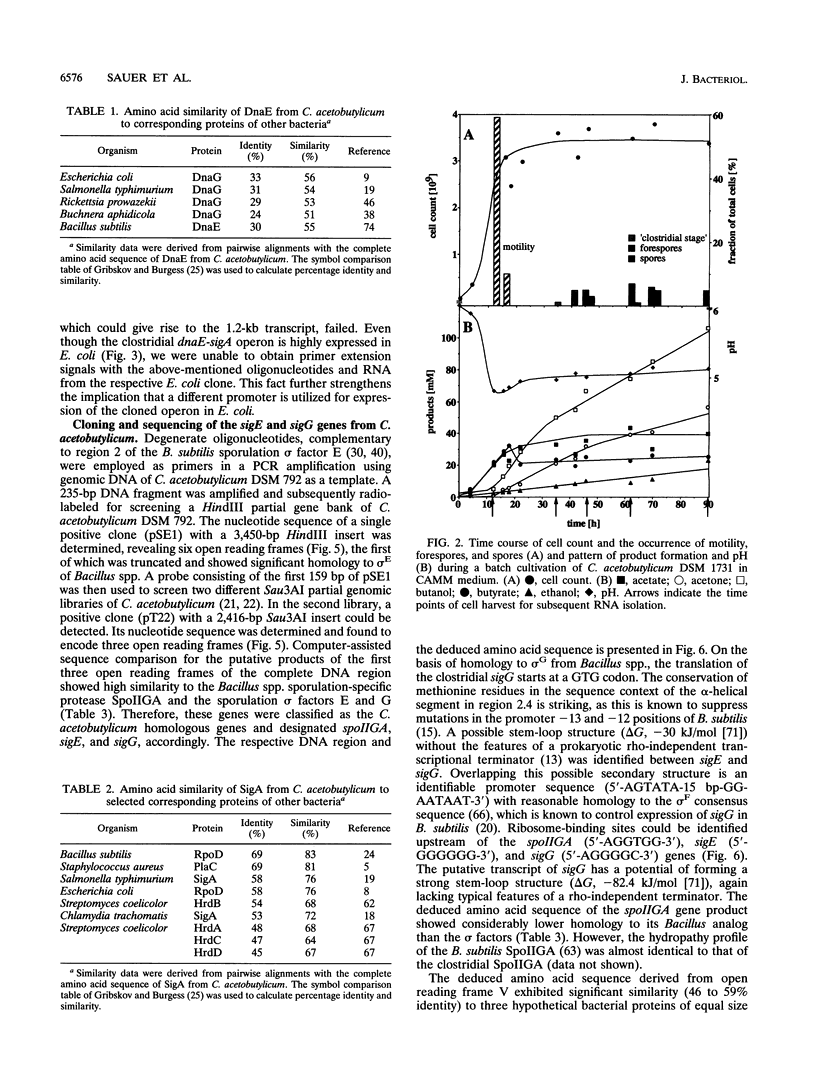
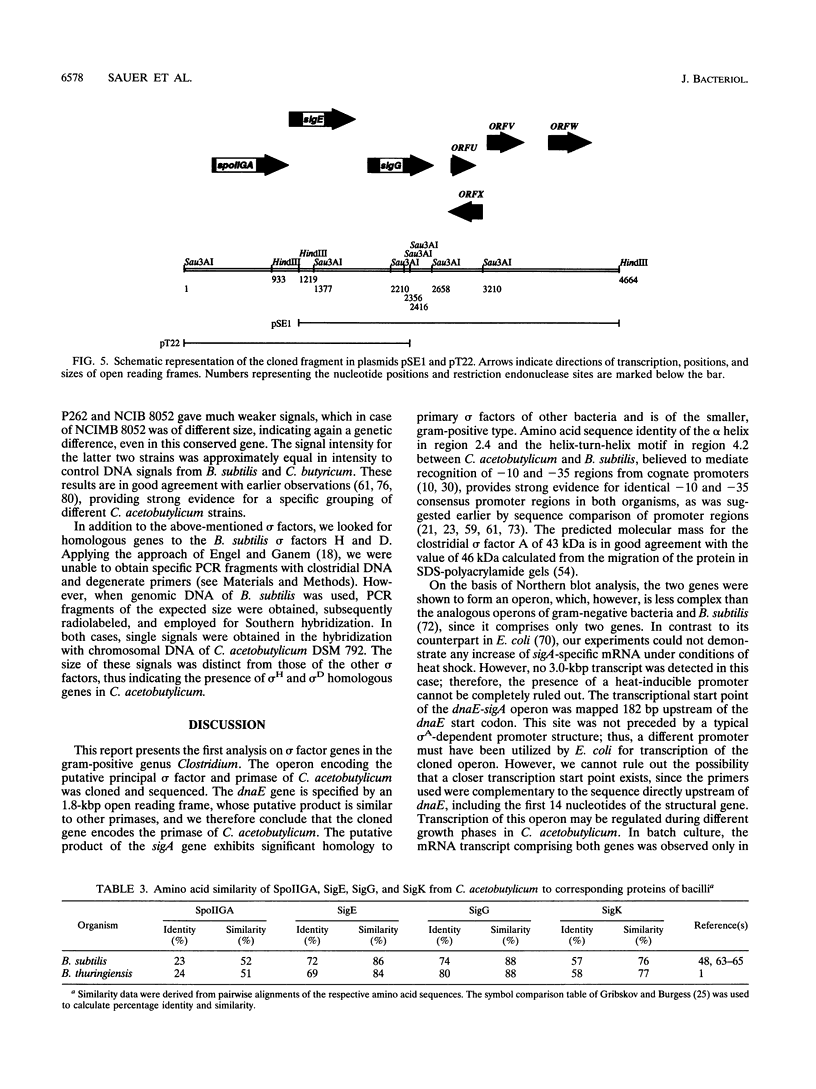
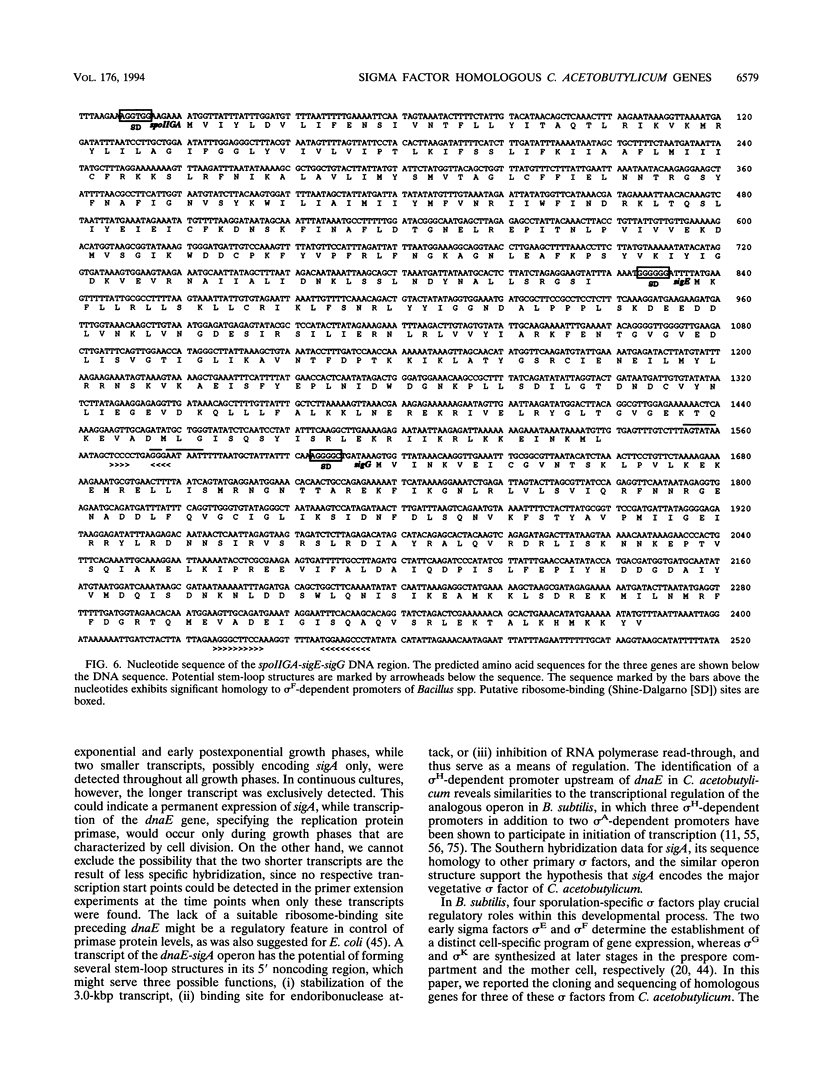
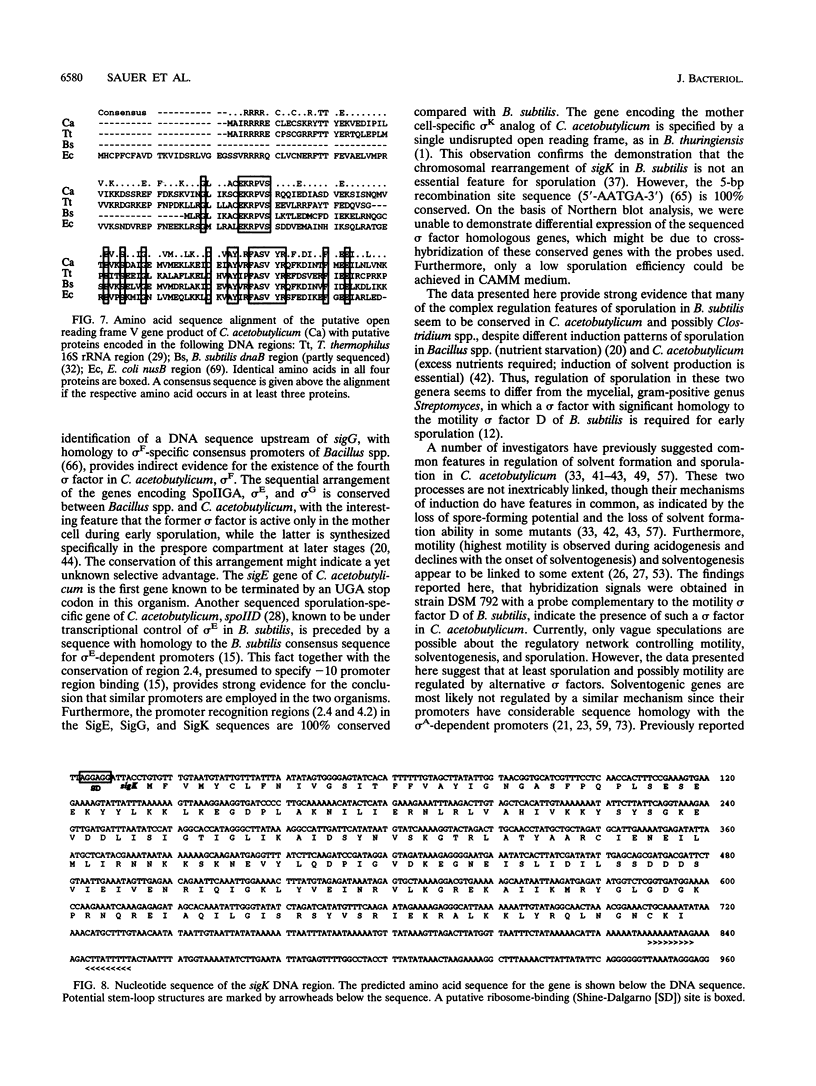
Using a PCR-based approach, we have cloned various sigma factor homologous genes from Clostridium acetobutylicum DSM 792. The nucleotide sequence of the dnaE-sigA operon has been determined and predicts two genes encoding 69- and 43-kDa proteins. The deduced DnaE amino acid sequence has approximately 30% amino acid identity with protein sequences of other primases. The putative sigA gene product shows high homology to primary sigma factors of various bacteria, most significantly to Bacillus subtilis and Staphylococcus aureus. Northern (RNA) blot analysis revealed that both genes from an operon, which is clearly expressed under conditions that allow for cell division. A promoter sequence with significant homology to the sigma H-dependent Bacillus promoters preceded the determined transcriptional start point, 182 bp upstream of the GUG start codon of dnaE. The homologous genes to Bacillus spp. sporulation sigma factors G, E, and K have been cloned and sequenced. Indirect evidence for the existence of sigma F was obtained by identification of a DNA sequence homologous to the respective Bacillus consensus promoter. Southern hybridization analysis indicated the presence of sigma D and sigma H homologous genes in C. acetobutylicum. A new gene group conserved within the eubacteria, but with yet unspecified functions, is described. The data presented here provide strong evidence that at least some of the complex regulation features of sporulation in B. subtilis are conserved in C. acetobutylicum and possibly Clostridium spp.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. F., Brown K. L., Whiteley H. R. Molecular cloning and characterization of two genes encoding sigma factors that direct transcription from a Bacillus thuringiensis crystal protein gene promoter. J Bacteriol. 1991 Jun;173(12):3846–3854. doi: 10.1128/jb.173.12.3846-3854.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basheer R., Iordanescu S. The Staphylococcus aureus chromosomal gene plaC, identified by mutations amplifying plasmid pT181, encodes a sigma factor. Nucleic Acids Res. 1991 Sep 25;19(18):4921–4924. doi: 10.1093/nar/19.18.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buluwela L., Forster A., Boehm T., Rabbitts T. H. A rapid procedure for colony screening using nylon filters. Nucleic Acids Res. 1989 Jan 11;17(1):452–452. doi: 10.1093/nar/17.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton Z. F., Gross C. A., Watanabe K. K., Burgess R. R. The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell. 1983 Feb;32(2):335–349. doi: 10.1016/0092-8674(83)90453-1. [DOI] [PubMed] [Google Scholar]
- Burton Z., Burgess R. R., Lin J., Moore D., Holder S., Gross C. A. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E coli K12. Nucleic Acids Res. 1981 Jun 25;9(12):2889–2903. doi: 10.1093/nar/9.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter H. L., 3rd, Wang L. F., Doi R. H., Moran C. P., Jr rpoD operon promoter used by sigma H-RNA polymerase in Bacillus subtilis. J Bacteriol. 1988 Apr;170(4):1617–1621. doi: 10.1128/jb.170.4.1617-1621.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B. Y., Doi R. H. Effects of amino acid substitutions in the promoter -10 binding region of the sigma A factor on growth of Bacillus subtilis. J Bacteriol. 1993 Apr;175(8):2470–2474. doi: 10.1128/jb.175.8.2470-2474.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater K. F., Bruton C. J., Plaskitt K. A., Buttner M. J., Méndez C., Helmann J. D. The developmental fate of S. coelicolor hyphae depends upon a gene product homologous with the motility sigma factor of B. subtilis. Cell. 1989 Oct 6;59(1):133–143. doi: 10.1016/0092-8674(89)90876-3. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich B., Tatti K. M., Jones C. H., Beall B., Moran C. P., Jr Genetic suppression analysis of sigma E interaction with three promoters in sporulating Bacillus subtilis. Gene. 1992 Nov 2;121(1):63–69. doi: 10.1016/0378-1119(92)90162-i. [DOI] [PubMed] [Google Scholar]
- Dombroski A. J., Walter W. A., Record M. T., Jr, Siegele D. A., Gross C. A. Polypeptides containing highly conserved regions of transcription initiation factor sigma 70 exhibit specificity of binding to promoter DNA. Cell. 1992 Aug 7;70(3):501–512. doi: 10.1016/0092-8674(92)90174-b. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. N., Ganem D. A polymerase chain reaction-based approach to cloning sigma factors from eubacteria and its application to the isolation of a sigma-70 homolog from Chlamydia trachomatis. J Bacteriol. 1990 May;172(5):2447–2455. doi: 10.1128/jb.172.5.2447-2455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson B. D., Burton Z. F., Watanabe K. K., Burgess R. R. Nucleotide sequence of the rpsU-dnaG-rpoD operon from Salmonella typhimurium and a comparison of this sequence with the homologous operon of Escherichia coli. Gene. 1985;40(1):67–78. doi: 10.1016/0378-1119(85)90025-3. [DOI] [PubMed] [Google Scholar]
- Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993 Mar;57(1):1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R. J., Helms J., Dürre P. Cloning, sequencing, and molecular analysis of the sol operon of Clostridium acetobutylicum, a chromosomal locus involved in solventogenesis. J Bacteriol. 1993 Nov;175(21):6959–6969. doi: 10.1128/jb.175.21.6959-6969.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerischer U., Dürre P. Cloning, sequencing, and molecular analysis of the acetoacetate decarboxylase gene region from Clostridium acetobutylicum. J Bacteriol. 1990 Dec;172(12):6907–6918. doi: 10.1128/jb.172.12.6907-6918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerischer U., Dürre P. mRNA analysis of the adc gene region of Clostridium acetobutylicum during the shift to solventogenesis. J Bacteriol. 1992 Jan;174(2):426–433. doi: 10.1128/jb.174.2.426-433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitt M. A., Wang L. F., Doi R. H. A strong sequence homology exists between the major RNA polymerase sigma factors of Bacillus subtilis and Escherichia coli. J Biol Chem. 1985 Jun 25;260(12):7178–7185. [PubMed] [Google Scholar]
- Gribskov M., Burgess R. R. Sigma factors from E. coli, B. subtilis, phage SP01, and phage T4 are homologous proteins. Nucleic Acids Res. 1986 Aug 26;14(16):6745–6763. doi: 10.1093/nar/14.16.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez N. A., Maddox I. S. Role of Chemotaxis in Solvent Production by Clostridium acetobutylicum. Appl Environ Microbiol. 1987 Aug;53(8):1924–1927. doi: 10.1128/aem.53.8.1924-1927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R. K., Erdmann V. A. Thermus thermophilus 16S rRNA is transcribed from an isolated transcription unit. J Bacteriol. 1989 Jun;171(6):2933–2941. doi: 10.1128/jb.171.6.2933-2941.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- Holck A., Blom H., Granum P. E. Cloning and sequencing of the genes encoding acid-soluble spore proteins from Clostridium perfringens. Gene. 1990 Jul 2;91(1):107–111. doi: 10.1016/0378-1119(90)90169-r. [DOI] [PubMed] [Google Scholar]
- Hoshino T., McKenzie T., Schmidt S., Tanaka T., Sueoka N. Nucleotide sequence of Bacillus subtilis dnaB: a gene essential for DNA replication initiation and membrane attachment. Proc Natl Acad Sci U S A. 1987 Feb;84(3):653–657. doi: 10.1073/pnas.84.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986 Dec;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., van der Westhuizen A., Long S., Allcock E. R., Reid S. J., Woods D. R. Solvent Production and Morphological Changes in Clostridium acetobutylicum. Appl Environ Microbiol. 1982 Jun;43(6):1434–1439. doi: 10.1128/aem.43.6.1434-1439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney T. J., Moran C. P., Jr Genetic evidence for interaction of sigma A with two promoters in Bacillus subtilis. J Bacteriol. 1991 Jun;173(11):3282–3290. doi: 10.1128/jb.173.11.3282-3290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormanec J., Farkasovský M., Potúcková L. Four genes in Streptomyces aureofaciens containing a domain characteristic of principal sigma factors. Gene. 1992 Dec 1;122(1):63–70. doi: 10.1016/0378-1119(92)90032-k. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Baumann P. Sequence analysis of a DNA fragment from Buchnera aphidicola (an endosymbiont of aphids) containing genes homologous to dnaG, rpoD, cysE, and secB. Gene. 1992 Sep 21;119(1):113–118. doi: 10.1016/0378-1119(92)90074-y. [DOI] [PubMed] [Google Scholar]
- Landuyt S. L., Hsu E. J. Preparation of Refractile Spores of Clostridium thermosaccharolyticum Involves a Solventogenic Phase. Appl Environ Microbiol. 1992 Jun;58(6):1797–1800. doi: 10.1128/aem.58.6.1797-1800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonetto M., Gribskov M., Gross C. A. The sigma 70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992 Jun;174(12):3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S., Jones D. T., Woods D. R. Sporulation of Clostridium acetobutylicum P262 in a Defined Medium. Appl Environ Microbiol. 1983 Apr;45(4):1389–1393. doi: 10.1128/aem.45.4.1389-1393.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Stragier P. Crisscross regulation of cell-type-specific gene expression during development in B. subtilis. Nature. 1992 Feb 13;355(6361):601–604. doi: 10.1038/355601a0. [DOI] [PubMed] [Google Scholar]
- Lupski J. R., Smiley B. L., Godson G. N. Regulation of the rpsU-dnaG-rpoD macromolecular synthesis operon and the initiation of DNA replication in Escherichia coli K-12. Mol Gen Genet. 1983;189(1):48–57. doi: 10.1007/BF00326054. [DOI] [PubMed] [Google Scholar]
- Marks G. L., Wood D. O. Characterization of the gene coding for the Rickettsia prowazekii DNA primase analogue. Gene. 1993 Jan 15;123(1):121–125. doi: 10.1016/0378-1119(93)90550-m. [DOI] [PubMed] [Google Scholar]
- Masuda E. S., Anaguchi H., Yamada K., Kobayashi Y. Two developmental genes encoding sigma factor homologs are arranged in tandem in Bacillus subtilis. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7637–7641. doi: 10.1073/pnas.85.20.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinecke B., Bahl H., Gottschalk G. Selection of an Asporogenous Strain of Clostridium acetobutylicum in Continuous Culture Under Phosphate Limitation. Appl Environ Microbiol. 1984 Nov;48(5):1064–1065. doi: 10.1128/aem.48.5.1064-1065.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971 Nov;68(3):307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- Pich A., Bahl H. Purification and characterization of the DNA-dependent RNA polymerase from Clostridium acetobutylicum. J Bacteriol. 1991 Mar;173(6):2120–2124. doi: 10.1128/jb.173.6.2120-2124.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F. X., Doi R. H. Localization of a second SigH promoter in the Bacillus subtilis sigA operon and regulation of dnaE expression by the promoter. J Bacteriol. 1990 Oct;172(10):5631–5636. doi: 10.1128/jb.172.10.5631-5636.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F. X., He X. S., Doi R. H. Localization of a new promoter, P5, in the sigA operon of Bacillus subtilis and its regulation in some spo mutant strains. J Bacteriol. 1991 Nov;173(21):7050–7054. doi: 10.1128/jb.173.21.7050-7054.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers P., Palosaari N. Clostridium acetobutylicum Mutants That Produce Butyraldehyde and Altered Quantities of Solvents. Appl Environ Microbiol. 1987 Dec;53(12):2761–2766. doi: 10.1128/aem.53.12.2761-2766.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer U., Dürre P. Possible function of tRNA(Thr)ACG in regulation of solvent formation in Clostridium acetobutylicum. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):147–153. doi: 10.1111/j.1574-6968.1992.tb14033.x. [DOI] [PubMed] [Google Scholar]
- Sauer U., Dürre P. Sequence and molecular characterization of a DNA region encoding a small heat shock protein of Clostridium acetobutylicum. J Bacteriol. 1993 Jun;175(11):3394–3400. doi: 10.1128/jb.175.11.3394-3400.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina T., Tanaka K., Takahashi H. Sequence of hrdB, an essential gene encoding sigma-like transcription factor of Streptomyces coelicolor A3(2): homology to principal sigma factors. Gene. 1991 Oct 30;107(1):145–148. doi: 10.1016/0378-1119(91)90308-x. [DOI] [PubMed] [Google Scholar]
- Stragier P., Bonamy C., Karmazyn-Campelli C. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell. 1988 Mar 11;52(5):697–704. doi: 10.1016/0092-8674(88)90407-2. [DOI] [PubMed] [Google Scholar]
- Stragier P., Bouvier J., Bonamy C., Szulmajster J. A developmental gene product of Bacillus subtilis homologous to the sigma factor of Escherichia coli. Nature. 1984 Nov 22;312(5992):376–378. doi: 10.1038/312376a0. [DOI] [PubMed] [Google Scholar]
- Stragier P., Kunkel B., Kroos L., Losick R. Chromosomal rearrangement generating a composite gene for a developmental transcription factor. Science. 1989 Jan 27;243(4890):507–512. doi: 10.1126/science.2536191. [DOI] [PubMed] [Google Scholar]
- Sun D., Fajardo-Cavazos P., Sussman M. D., Tovar-Rojo F., Cabrera-Martinez R. M., Setlow P. Effect of chromosome location of Bacillus subtilis forespore genes on their spo gene dependence and transcription by E sigma F: identification of features of good E sigma F-dependent promoters. J Bacteriol. 1991 Dec;173(24):7867–7874. doi: 10.1128/jb.173.24.7867-7874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Shiina T., Takahashi H. Nucleotide sequence of genes hrdA, hrdC, and hrdD from Streptomyces coelicolor A3(2) having similarity to rpoD genes. Mol Gen Genet. 1991 Oct;229(3):334–340. doi: 10.1007/BF00267453. [DOI] [PubMed] [Google Scholar]
- Tatti K. M., Carter H. L., 3rd, Moir A., Moran C. P., Jr Sigma H-directed transcription of citG in Bacillus subtilis. J Bacteriol. 1989 Nov;171(11):5928–5932. doi: 10.1128/jb.171.11.5928-5932.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taura T., Ueguchi C., Shiba K., Ito K. Insertional disruption of the nusB (ssyB) gene leads to cold-sensitive growth of Escherichia coli and suppression of the secY24 mutation. Mol Gen Genet. 1992 Sep;234(3):429–432. doi: 10.1007/BF00538702. [DOI] [PubMed] [Google Scholar]
- Taylor W. E., Straus D. B., Grossman A. D., Burton Z. F., Gross C. A., Burgess R. R. Transcription from a heat-inducible promoter causes heat shock regulation of the sigma subunit of E. coli RNA polymerase. Cell. 1984 Sep;38(2):371–381. doi: 10.1016/0092-8674(84)90492-6. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Versalovic J., Koeuth T., Britton R., Geszvain K., Lupski J. R. Conservation and evolution of the rpsU-dnaG-rpoD macromolecular synthesis operon in bacteria. Mol Microbiol. 1993 Apr;8(2):343–355. doi: 10.1111/j.1365-2958.1993.tb01578.x. [DOI] [PubMed] [Google Scholar]
- Walter K. A., Bennett G. N., Papoutsakis E. T. Molecular characterization of two Clostridium acetobutylicum ATCC 824 butanol dehydrogenase isozyme genes. J Bacteriol. 1992 Nov;174(22):7149–7158. doi: 10.1128/jb.174.22.7149-7158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. F., Doi R. H. Nucleotide sequence and organization of Bacillus subtilis RNA polymerase major sigma (sigma 43) operon. Nucleic Acids Res. 1986 May 27;14(10):4293–4307. doi: 10.1093/nar/14.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. F., Doi R. H. Promoter switching during development and the termination site of the sigma 43 operon of Bacillus subtilis. Mol Gen Genet. 1987 Apr;207(1):114–119. doi: 10.1007/BF00331498. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young M., Minton N. P., Staudenbauer W. L. Recent advances in the genetics of the clostridia. FEMS Microbiol Rev. 1989 Dec;5(4):301–325. doi: 10.1111/j.1574-6968.1989.tb03402.x. [DOI] [PubMed] [Google Scholar]
- d'Aubenton Carafa Y., Brody E., Thermes C. Prediction of rho-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem-loop structures. J Mol Biol. 1990 Dec 20;216(4):835–858. doi: 10.1016/s0022-2836(99)80005-9. [DOI] [PubMed] [Google Scholar]