Abstract
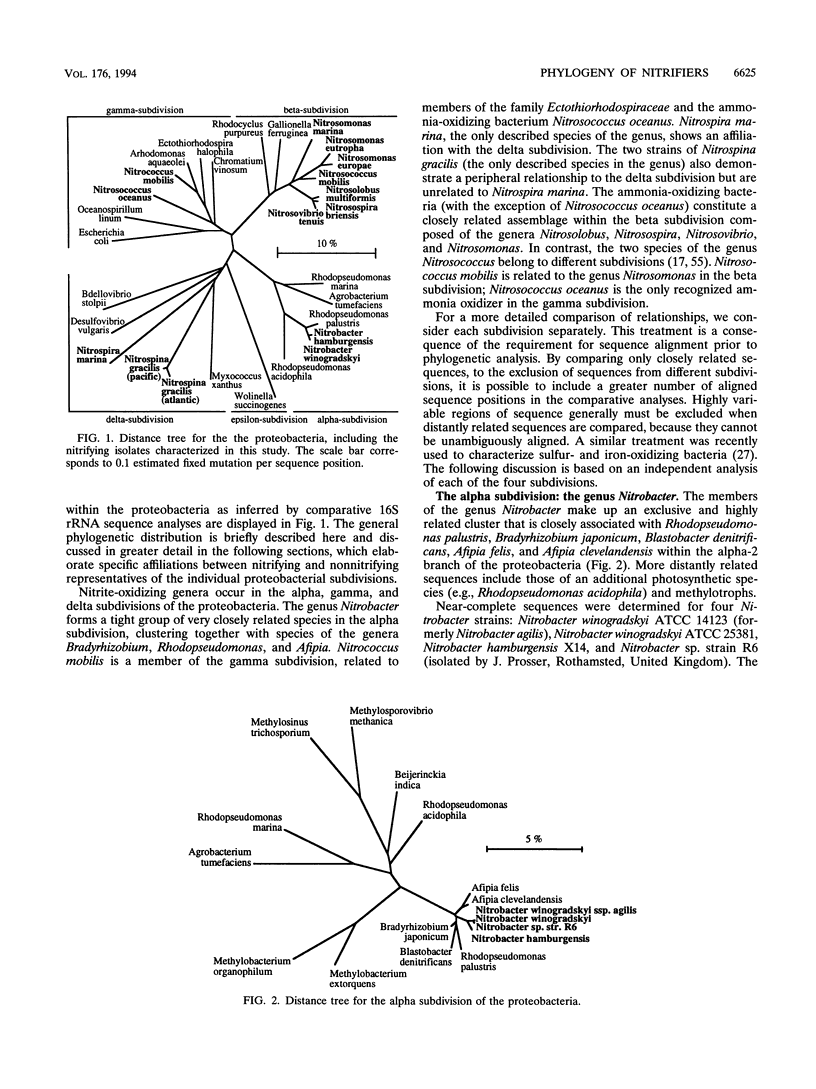
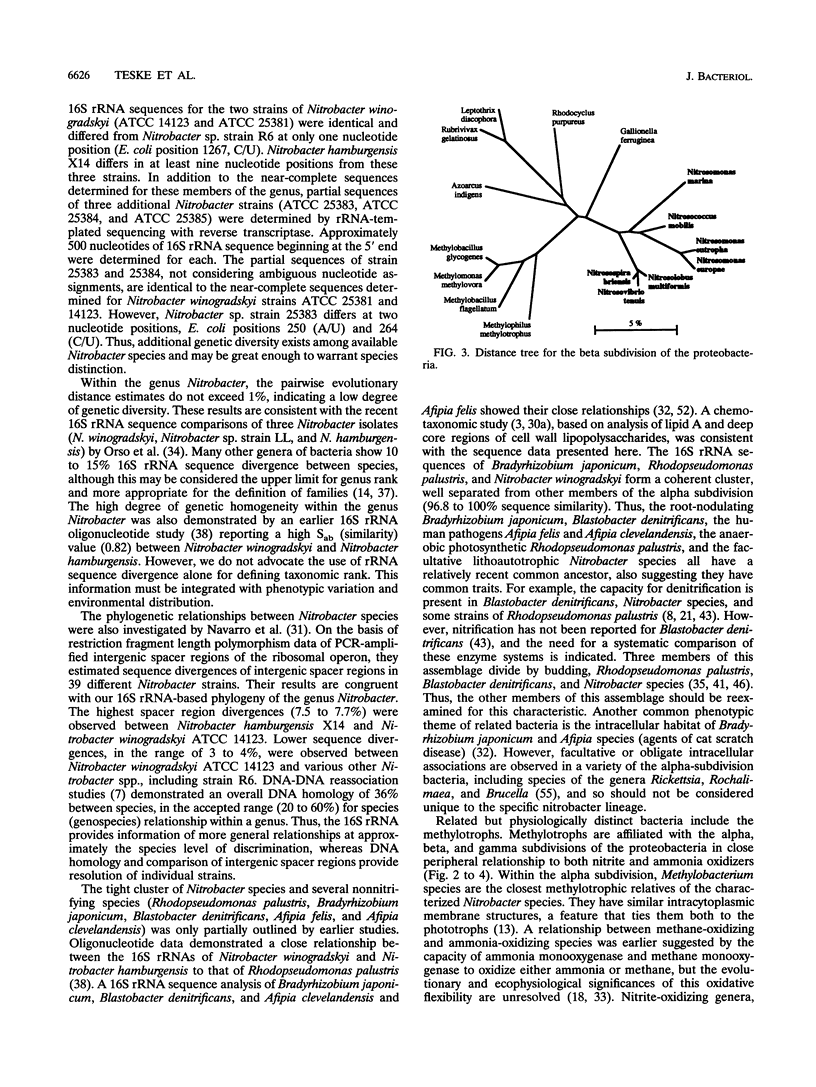
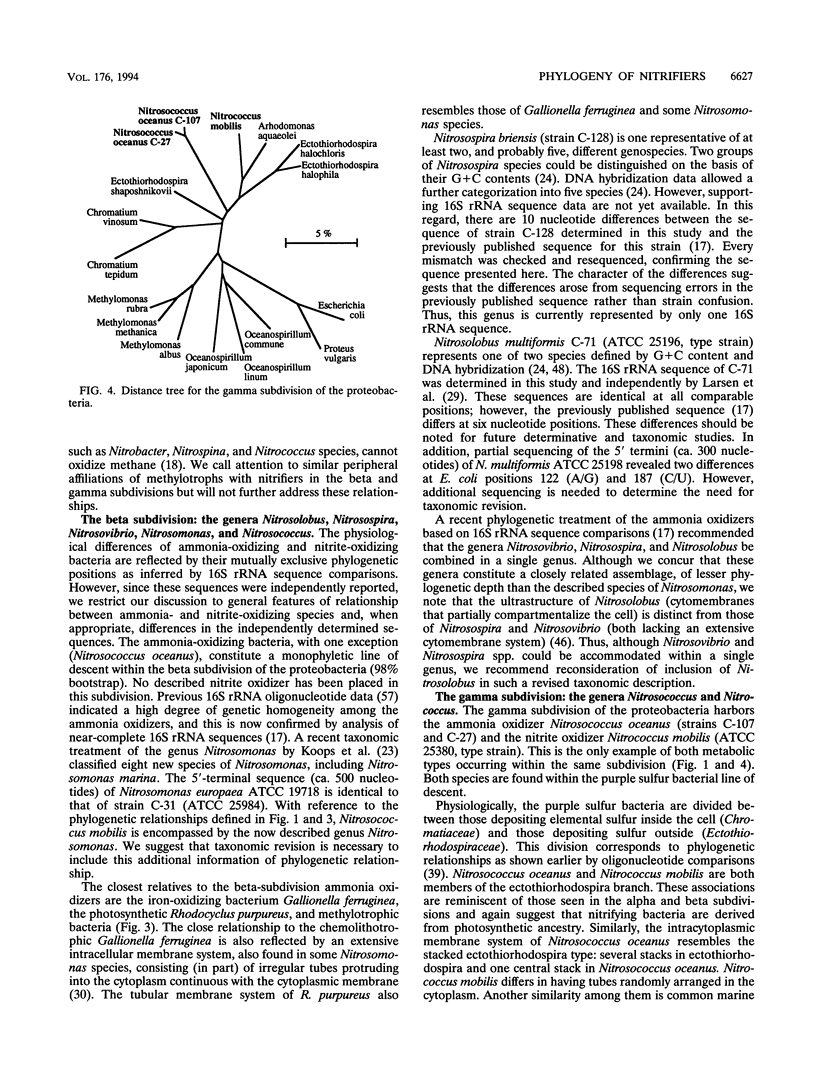
Comparative 16S rRNA sequencing was used to evaluate phylogenetic relationships among selected strains of ammonia- and nitrite-oxidizing bacteria. All characterized strains were shown to be affiliated with the proteobacteria. The study extended recent 16S rRNA-based studies of phylogenetic diversity among nitrifiers by the comparison of eight strains of the genus Nitrobacter and representatives of the genera Nitrospira and Nitrospina. The later genera were shown to be affiliated with the delta subdivision of the proteobacteria but did not share a specific relationship to each other or to other members of the delta subdivision. All characterized Nitrobacter strains constituted a closely related assemblage within the alpha subdivision of the proteobacteria. As previously observed, all ammonia-oxidizing genera except Nitrosococcus oceanus constitute a monophyletic assemblage within the beta subdivision of the proteobacteria. Errors in the 16S rRNA sequences for two strains previously deposited in the databases by other investigators (Nitrosolobus multiformis C-71 and Nitrospira briensis C-128) were corrected. Consideration of physiology and phylogenetic distribution suggested that nitrite-oxidizing bacteria of the alpha and gamma subdivisions are derived from immediate photosynthetic ancestry. Each nitrifier retains the general structural features of the specific ancestor's photosynthetic membrane complex. Thus, the nitrifiers, as a group, apparently are not derived from an ancestral nitrifying phenotype.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins J. P., Madigan M. T., Mandelco L., Woese C. R., Tanner R. S. Arhodomonas aquaeolei gen. nov., sp. nov., an aerobic, halophilic bacterium isolated from a subterranean brine. Int J Syst Bacteriol. 1993 Jul;43(3):514–520. doi: 10.1099/00207713-43-3-514. [DOI] [PubMed] [Google Scholar]
- Amann R. I., Binder B. J., Olson R. J., Chisholm S. W., Devereux R., Stahl D. A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990 Jun;56(6):1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer M., Chase T., Watson S. W. Fatty acids in the lipids of marine and terrestrial nitrifying bacteria. J Bacteriol. 1969 Aug;99(2):366–370. doi: 10.1128/jb.99.2.366-370.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broda E., Peschek G. A. Did respiration or photosynthesis come first? J Theor Biol. 1979 Nov 21;81(2):201–212. doi: 10.1016/0022-5193(79)90160-7. [DOI] [PubMed] [Google Scholar]
- Broda E. Two kinds of lithotrophs missing in nature. Z Allg Mikrobiol. 1977;17(6):491–493. doi: 10.1002/jobm.3630170611. [DOI] [PubMed] [Google Scholar]
- Böck A., Stadtman T. C. Selenocysteine, a highly specific component of certain enzymes, is incorporated by a UGA-directed co-translational mechanism. Biofactors. 1988 Oct;1(3):245–250. [PubMed] [Google Scholar]
- Böttger E. C. Rapid determination of bacterial ribosomal RNA sequences by direct sequencing of enzymatically amplified DNA. FEMS Microbiol Lett. 1989 Nov;53(1-2):171–176. doi: 10.1016/0378-1097(89)90386-8. [DOI] [PubMed] [Google Scholar]
- Davies S. L., Whittenbury R. Fine structure of methane and other hydrocarbon-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):227–232. doi: 10.1099/00221287-61-2-227. [DOI] [PubMed] [Google Scholar]
- Devereux R., He S. H., Doyle C. L., Orkland S., Stahl D. A., LeGall J., Whitman W. B. Diversity and origin of Desulfovibrio species: phylogenetic definition of a family. J Bacteriol. 1990 Jul;172(7):3609–3619. doi: 10.1128/jb.172.7.3609-3619.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms H., Koops H. P., Wehrmann H. An ammonia-oxidizing bacterium, Nitrosovibrio tenuis nov. gen. nov. sp. Arch Microbiol. 1976 May 3;108(1):105–111. doi: 10.1007/BF00425099. [DOI] [PubMed] [Google Scholar]
- Head I. M., Hiorns W. D., Embley T. M., McCarthy A. J., Saunders J. R. The phylogeny of autotrophic ammonia-oxidizing bacteria as determined by analysis of 16S ribosomal RNA gene sequences. J Gen Microbiol. 1993 Jun;139(Pt 6):1147–1153. doi: 10.1099/00221287-139-6-1147. [DOI] [PubMed] [Google Scholar]
- Jones R. D., Morita R. Y. Methane Oxidation by Nitrosococcus oceanus and Nitrosomonas europaea. Appl Environ Microbiol. 1983 Feb;45(2):401–410. doi: 10.1128/aem.45.2.401-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koops H. P., Harms H. Deoxyribonucleic acid homologies among 96 strains of ammonia-oxidizing bacteria. Arch Microbiol. 1985 Apr;141(3):214–218. doi: 10.1007/BF00408061. [DOI] [PubMed] [Google Scholar]
- Koops H. P., Harms H., Wehrmann H. Isolation of a moderate halophilic ammonia-oxidizing bacterium, Nitrosococcus mobilis nov. sp. Arch Microbiol. 1976 Apr 1;107(3):277–282. doi: 10.1007/BF00425339. [DOI] [PubMed] [Google Scholar]
- Lane D. J., Harrison A. P., Jr, Stahl D., Pace B., Giovannoni S. J., Olsen G. J., Pace N. R. Evolutionary relationships among sulfur- and iron-oxidizing eubacteria. J Bacteriol. 1992 Jan;174(1):269–278. doi: 10.1128/jb.174.1.269-278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. J., Pace B., Olsen G. J., Stahl D. A., Sogin M. L., Pace N. R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen N., Olsen G. J., Maidak B. L., McCaughey M. J., Overbeek R., Macke T. J., Marsh T. L., Woese C. R. The ribosomal database project. Nucleic Acids Res. 1993 Jul 1;21(13):3021–3023. doi: 10.1093/nar/21.13.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer H., Krauss J. H., Urbanik-Sypniewska T., Puvanesarajah V., Stacey G., Auling G. Lipid A with 2,3-diamino-2,3-dideoxy-glucose in lipopolysaccharides from slow-growing members of Rhizobiaceae and from "Pseudomonas carboxydovorans". Arch Microbiol. 1989;151(2):111–116. doi: 10.1007/BF00414423. [DOI] [PubMed] [Google Scholar]
- Navarro E., Simonet P., Normand P., Bardin R. Characterization of natural populations of Nitrobacter spp. using PCR/RFLP analysis of the ribosomal intergenic spacer. Arch Microbiol. 1992;157(2):107–115. doi: 10.1007/BF00245277. [DOI] [PubMed] [Google Scholar]
- O'Connor S. P., Dorsch M., Steigerwalt A. G., Brenner D. J., Stackebrandt E. 16S rRNA sequences of Bartonella bacilliformis and cat scratch disease bacillus reveal phylogenetic relationships with the alpha-2 subgroup of the class Proteobacteria. J Clin Microbiol. 1991 Oct;29(10):2144–2150. doi: 10.1128/jcm.29.10.2144-2150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orso S., Gouy M., Navarro E., Normand P. Molecular phylogenetic analysis of Nitrobacter spp. Int J Syst Bacteriol. 1994 Jan;44(1):83–86. doi: 10.1099/00207713-44-1-83. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Stackebrandt E. Molecular systematics of prokaryotes. Annu Rev Microbiol. 1983;37:143–187. doi: 10.1146/annurev.mi.37.100183.001043. [DOI] [PubMed] [Google Scholar]
- Stahl D. A., Flesher B., Mansfield H. R., Montgomery L. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol. 1988 May;54(5):1079–1084. doi: 10.1128/aem.54.5.1079-1084.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Graham L. B., Remsen C. C., Valois F. W. A lobular, ammonia-oxidizing bacterium, Nitrosolobus multiformis nov.gen.nov.sp. Arch Mikrobiol. 1971;76(3):183–203. doi: 10.1007/BF00409115. [DOI] [PubMed] [Google Scholar]
- Watson S. W., Mandel M. Comparison of the morphology and deoxyribonucleic acid composition of 27 strains of nitrifying bacteria. J Bacteriol. 1971 Aug;107(2):563–569. doi: 10.1128/jb.107.2.563-569.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems A., Collins M. D. Evidence for a close genealogical relationship between Afipia (the causal organism of cat scratch disease), Bradyrhizobium japonicum and Blastobacter denitrificans. FEMS Microbiol Lett. 1992 Sep 15;75(2-3):241–246. doi: 10.1016/0378-1097(92)90411-g. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Weisburg W. G., Paster B. J., Madigan M. T., Fowler V. J., Hahn C. M., Blanz P., Gupta R., Nealson K. H. The phylogeny of purple bacteria: the alpha subdivision. Syst Appl Microbiol. 1984;5:315–326. doi: 10.1016/s0723-2020(84)80034-x. [DOI] [PubMed] [Google Scholar]


