Abstract
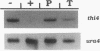
thi4 mutants of Schizosaccharomyces pombe exhibit defective thiamine biosynthesis, and thi4 mutations define a gene which is believed to be involved in the phosphorylation of 4-amino-5-hydroxymethyl-2-methylpyrimidine or 5-(2-hydroxyethyl)-4-methylthiazole and/or in the coupling of the two phosphorylated precursors to thiamine monophosphate (A. M. Schweingruber, J. Dlugonski, E. Edenharter, and M. E. Schweingruber, Curr. Genet. 19:249-254, 1991). The thi4 gene was cloned by functional complementation of a thi4 mutant and physically mapped on the left arm of chromosome I close to the genetic marker gln1. The thi4-carrying DNA fragment shows an open reading frame encoding a protein of 518 amino acids and a calculated molecular mass of 55.6 kDa. The appearance of thi4 mRNA is strongly repressed by thiamine and to a lesser extent by 5-(2-hydroxyethyl)-4-methylthiazole. thi4 mRNA production is under the control of the thi1 gene-encoded transcription factor and of the negative regulators encoded by genes tnr1, tnr2, and tnr3. thi4 is expressed and regulated in manners similar to those of other S. pombe genes involved in thiamine metabolism, including thi2, thi3, and pho4.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basi G., Schmid E., Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993 Jan 15;123(1):131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Elliott S., Chang C. W., Schweingruber M. E., Schaller J., Rickli E. E., Carbon J. Isolation and characterization of the structural gene for secreted acid phosphatase from Schizosaccharomyces pombe. J Biol Chem. 1986 Feb 25;261(6):2936–2941. [PubMed] [Google Scholar]
- Fankhauser H., Schweingruber M. E. Thiamine-repressible genes in Schizosaccharomyces pombe are regulated by a Cys6 zinc-finger motif-containing protein. Gene. 1994 Sep 15;147(1):141–144. doi: 10.1016/0378-1119(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Hoheisel J. D., Maier E., Mott R., McCarthy L., Grigoriev A. V., Schalkwyk L. C., Nizetic D., Francis F., Lehrach H. High resolution cosmid and P1 maps spanning the 14 Mb genome of the fission yeast S. pombe. Cell. 1993 Apr 9;73(1):109–120. doi: 10.1016/0092-8674(93)90164-l. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losson R., Lacroute F. Plasmids carrying the yeast OMP decarboxylase structural and regulatory genes: transcription regulation in a foreign environment. Cell. 1983 Feb;32(2):371–377. doi: 10.1016/0092-8674(83)90456-7. [DOI] [PubMed] [Google Scholar]
- Maier E., Hoheisel J. D., McCarthy L., Mott R., Grigoriev A. V., Monaco A. P., Larin Z., Lehrach H. Complete coverage of the Schizosaccharomyces pombe genome in yeast artificial chromosomes. Nat Genet. 1992 Jul;1(4):273–277. doi: 10.1038/ng0792-273. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990 Jul 5;265(19):10857–10864. [PubMed] [Google Scholar]
- Praekelt U. M., Byrne K. L., Meacock P. A. Regulation of THI4 (MOL1), a thiamine-biosynthetic gene of Saccharomyces cerevisiae. Yeast. 1994 Apr;10(4):481–490. doi: 10.1002/yea.320100407. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweingruber A. M., Dlugonski J., Edenharter E., Schweingruber M. E. Thiamine in Schizosaccharomyces pombe: dephosphorylation, intracellular pool, biosynthesis and transport. Curr Genet. 1991 Apr;19(4):249–254. doi: 10.1007/BF00355050. [DOI] [PubMed] [Google Scholar]
- Schweingruber A. M., Fankhauser H., Dlugonski J., Steinmann-Loss C., Schweingruber M. E. Isolation and characterization of regulatory mutants from Schizosaccharomyces pombe involved in thiamine-regulated gene expression. Genetics. 1992 Mar;130(3):445–449. doi: 10.1093/genetics/130.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweingruber M. E., Edenharter E. Thiamin regulates agglutination and zygote formation in Schizosaccharomyces pombe. Curr Genet. 1990 Mar;17(3):191–194. doi: 10.1007/BF00312609. [DOI] [PubMed] [Google Scholar]
- Schweingruber M. E., Fluri R., Maundrell K., Schweingruber A. M., Dumermuth E. Identification and characterization of thiamin repressible acid phosphatase in yeast. J Biol Chem. 1986 Dec 5;261(34):15877–15882. [PubMed] [Google Scholar]
- Vander Horn P. B., Backstrom A. D., Stewart V., Begley T. P. Structural genes for thiamine biosynthetic enzymes (thiCEFGH) in Escherichia coli K-12. J Bacteriol. 1993 Feb;175(4):982–992. doi: 10.1128/jb.175.4.982-992.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. W. The biosynthesis of the vitamins thiamin, riboflavin, and folic acid. Nat Prod Rep. 1986 Aug;3(4):395–419. doi: 10.1039/np9860300395. [DOI] [PubMed] [Google Scholar]
- Zurlinden A., Schweingruber M. E. Cloning and regulation of Schizosaccharomyces pombe thi2, a gene involved in thiamine biosynthesis. Gene. 1992 Aug 1;117(1):141–143. doi: 10.1016/0378-1119(92)90503-h. [DOI] [PubMed] [Google Scholar]