Abstract
Ribonucleotide reductase, an allosterically regulated, cell cycle-dependent enzyme catalyzing a unique step in the synthesis of DNA, the reduction of 2'-ribonucleotides to 2'-deoxyribonucleotides, was purified 500-fold from Mycobacterium tuberculosis Erdman strain through cell disruption, ammonium sulfate fractionation, and dATP-Sepharose affinity column chromatography. As in eucaryotes and certain bacteria and viruses, the M. tuberculosis enzyme consists of two nonidentical subunits, R1 and R2, both of which are required for activity. R1 has a molecular mass of 84 kDa, as identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and photoaffinity labeling with dATP. The amino acid sequences of the N-terminal peptide and two internal peptides were determined, and a partial R1 gene was isolated by PCR with primers designed from these amino acid sequences. Additional coding sequences were isolated by screening size-selected libraries, and a full-length form of M. tuberculosis R1 was generated by PCR amplification of high-molecular-weight M. tuberculosis DNA and expressed in Eschericnia coli. This coding sequence is 2,169 nucleotides long and contains no introns. The predicted molecular mass of R1 from the DNA sequence is 82,244 Da. Recombinant M. tuberculosis R1, purified to homogeneity, was biochemically active when assayed with extracts of M. tuberculosis enriched for R2.
Full text
PDF



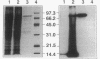
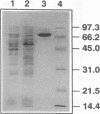
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alland D., Kalkut G. E., Moss A. R., McAdam R. A., Hahn J. A., Bosworth W., Drucker E., Bloom B. R. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994 Jun 16;330(24):1710–1716. doi: 10.1056/NEJM199406163302403. [DOI] [PubMed] [Google Scholar]
- Berglund O., Eckstein F. Synthesis of ATP- and dATP-substituted sepharoses and their application in the purification of phage-T4-induced ribonucleotide reductase. Eur J Biochem. 1972 Aug 4;28(4):492–496. doi: 10.1111/j.1432-1033.1972.tb01936.x. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Martin D. W., Jr Direct photoaffinity labeling of an allosteric site of subunit protein M1 of mouse ribonucleotide reductase by dATP. Evidence for two independent binding interactions within the allosteric specificity site. J Biol Chem. 1982 Aug 25;257(16):9508–9512. [PubMed] [Google Scholar]
- Carlson J., Fuchs J. A., Messing J. Primary structure of the Escherichia coli ribonucleoside diphosphate reductase operon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4294–4297. doi: 10.1073/pnas.81.14.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström Y., Eriksson S., Thelander L., Akerman M. Ribonucleotide reductase from calf thymus. Purification and properties. Biochemistry. 1979 Jul 10;18(14):2941–2948. doi: 10.1021/bi00581a004. [DOI] [PubMed] [Google Scholar]
- Mao S. S., Holler T. P., Bollinger J. M., Jr, Yu G. X., Johnston M. I., Stubbe J. Interaction of C225SR1 mutant subunit of ribonucleotide reductase with R2 and nucleoside diphosphates: tales of a suicidal enzyme. Biochemistry. 1992 Oct 13;31(40):9744–9751. doi: 10.1021/bi00155a030. [DOI] [PubMed] [Google Scholar]
- Mao S. S., Holler T. P., Yu G. X., Bollinger J. M., Jr, Booker S., Johnston M. I., Stubbe J. A model for the role of multiple cysteine residues involved in ribonucleotide reduction: amazing and still confusing. Biochemistry. 1992 Oct 13;31(40):9733–9743. doi: 10.1021/bi00155a029. [DOI] [PubMed] [Google Scholar]
- Mao S. S., Johnston M. I., Bollinger J. M., Stubbe J. Mechanism-based inhibition of a mutant Escherichia coli ribonucleotide reductase (cysteine-225----serine) by its substrate CDP. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1485–1489. doi: 10.1073/pnas.86.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S. S., Yu G. X., Chalfoun D., Stubbe J. Characterization of C439SR1, a mutant of Escherichia coli ribonucleotide diphosphate reductase: evidence that C439 is a residue essential for nucleotide reduction and C439SR1 is a protein possessing novel thioredoxin-like activity. Biochemistry. 1992 Oct 13;31(40):9752–9759. doi: 10.1021/bi00155a031. [DOI] [PubMed] [Google Scholar]
- Moore E. C., Peterson D., Yang L. Y., Yeung C. Y., Neff N. F. Separation of ribonucleotides and deoxyribonucleotides on columns of borate covalently linked to cellulose. Application to the assay of ribonucleoside diphosphate reductase. Biochemistry. 1974 Jul 2;13(14):2904–2907. doi: 10.1021/bi00711a020. [DOI] [PubMed] [Google Scholar]
- Moss N., Déziel R., Adams J., Aubry N., Bailey M., Baillet M., Beaulieu P., DiMaio J., Duceppe J. S., Ferland J. M. Inhibition of herpes simplex virus type 1 ribonucleotide reductase by substituted tetrapeptide derivatives. J Med Chem. 1993 Oct 1;36(20):3005–3009. doi: 10.1021/jm00072a021. [DOI] [PubMed] [Google Scholar]
- Reichard P. From RNA to DNA, why so many ribonucleotide reductases? Science. 1993 Jun 18;260(5115):1773–1777. doi: 10.1126/science.8511586. [DOI] [PubMed] [Google Scholar]
- Rubin H., Plotnick M., Wang Z. M., Liu X., Zhong Q., Schechter N. M., Cooperman B. S. Conversion of alpha 1-antichymotrypsin into a human neutrophil elastase inhibitor: demonstration of variants with different association rate constants, stoichiometries of inhibition, and complex stabilities. Biochemistry. 1994 Jun 21;33(24):7627–7633. doi: 10.1021/bi00190a016. [DOI] [PubMed] [Google Scholar]
- Rubin H., Salem J. S., Li L. S., Yang F. D., Mama S., Wang Z. M., Fisher A., Hamann C. S., Cooperman B. S. Cloning, sequence determination, and regulation of the ribonucleotide reductase subunits from Plasmodium falciparum: a target for antimalarial therapy. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9280–9284. doi: 10.1073/pnas.90.20.9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Wang Z. M., Nickbarg E. B., McLarney S., Naidoo N., Schoenberger O. L., Johnson J. L., Cooperman B. S. Cloning, expression, purification, and biological activity of recombinant native and variant human alpha 1-antichymotrypsins. J Biol Chem. 1990 Jan 15;265(2):1199–1207. [PubMed] [Google Scholar]
- Schaper K. J., Seydel J. K., Rosenfeld M., Kazda J. Development of inhibitors of mycobacterial ribonucleotide reductase. Lepr Rev. 1986;57 (Suppl 3):254–264. doi: 10.5935/0305-7518.19860115. [DOI] [PubMed] [Google Scholar]
- Small P. M., Hopewell P. C., Singh S. P., Paz A., Parsonnet J., Ruston D. C., Schecter G. F., Daley C. L., Schoolnik G. K. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N Engl J Med. 1994 Jun 16;330(24):1703–1709. doi: 10.1056/NEJM199406163302402. [DOI] [PubMed] [Google Scholar]
- Steeper J. R., Steuart C. D. A rapid assay for CDP reductase activity in mammalian cell extracts. Anal Biochem. 1970 Mar;34:123–130. doi: 10.1016/0003-2697(70)90092-8. [DOI] [PubMed] [Google Scholar]
- Sun L., Jacobson B. A., Dien B. S., Srienc F., Fuchs J. A. Cell cycle regulation of the Escherichia coli nrd operon: requirement for a cis-acting upstream AT-rich sequence. J Bacteriol. 1994 Apr;176(8):2415–2426. doi: 10.1128/jb.176.8.2415-2426.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L. Physicochemical characterization of ribonucleoside diphosphate reductase from Escherichia coli. J Biol Chem. 1973 Jul 10;248(13):4591–4601. [PubMed] [Google Scholar]
- Thelander L., Sjöberg B. R., Eriksson S. Ribonucleoside diphosphate reductase (Escherichia coli). Methods Enzymol. 1978;51:227–237. doi: 10.1016/s0076-6879(78)51032-x. [DOI] [PubMed] [Google Scholar]
- Tuggle C. K., Fuchs J. A. Regulation of the operon encoding ribonucleotide reductase in Escherichia coli: evidence for both positive and negative control. EMBO J. 1986 May;5(5):1077–1085. doi: 10.1002/j.1460-2075.1986.tb04325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin U., Eklund H. Structure of ribonucleotide reductase protein R1. Nature. 1994 Aug 18;370(6490):533–539. doi: 10.1038/370533a0. [DOI] [PubMed] [Google Scholar]
- Wheeler P. R. Biosynthesis and scavenging of purines by pathogenic mycobacteria including Mycobacterium leprae. J Gen Microbiol. 1987 Nov;133(11):2999–3011. doi: 10.1099/00221287-133-11-2999. [DOI] [PubMed] [Google Scholar]
- Wheeler P. R. Biosynthesis and scavenging of pyrimidines by pathogenic mycobacteria. J Gen Microbiol. 1990 Jan;136(1):189–201. doi: 10.1099/00221287-136-1-189. [DOI] [PubMed] [Google Scholar]
- Wheeler P. R. Enzymes for purine synthesis and scavenging in pathogenic mycobacteria and their distribution in Mycobacterium leprae. J Gen Microbiol. 1987 Nov;133(11):3013–3018. doi: 10.1099/00221287-133-11-3013. [DOI] [PubMed] [Google Scholar]
- Winder F. G., Barber D. S. Effects of hydroxyurea, nalidixic acid and zinc limitation on DNA polymerase and ATP-dependent deoxyribonuclease activities of Mycobacterium smegmatis. J Gen Microbiol. 1973 May;76(1):189–196. doi: 10.1099/00221287-76-1-189. [DOI] [PubMed] [Google Scholar]
- Winder F. G., McNulty M. S. Increased DNA polymerase activity accompanying decreased DNA content in iron-deficient Mycobacterium smegmatis. Biochim Biophys Acta. 1970;209(2):578–580. doi: 10.1016/0005-2787(70)90757-4. [DOI] [PubMed] [Google Scholar]