Abstract
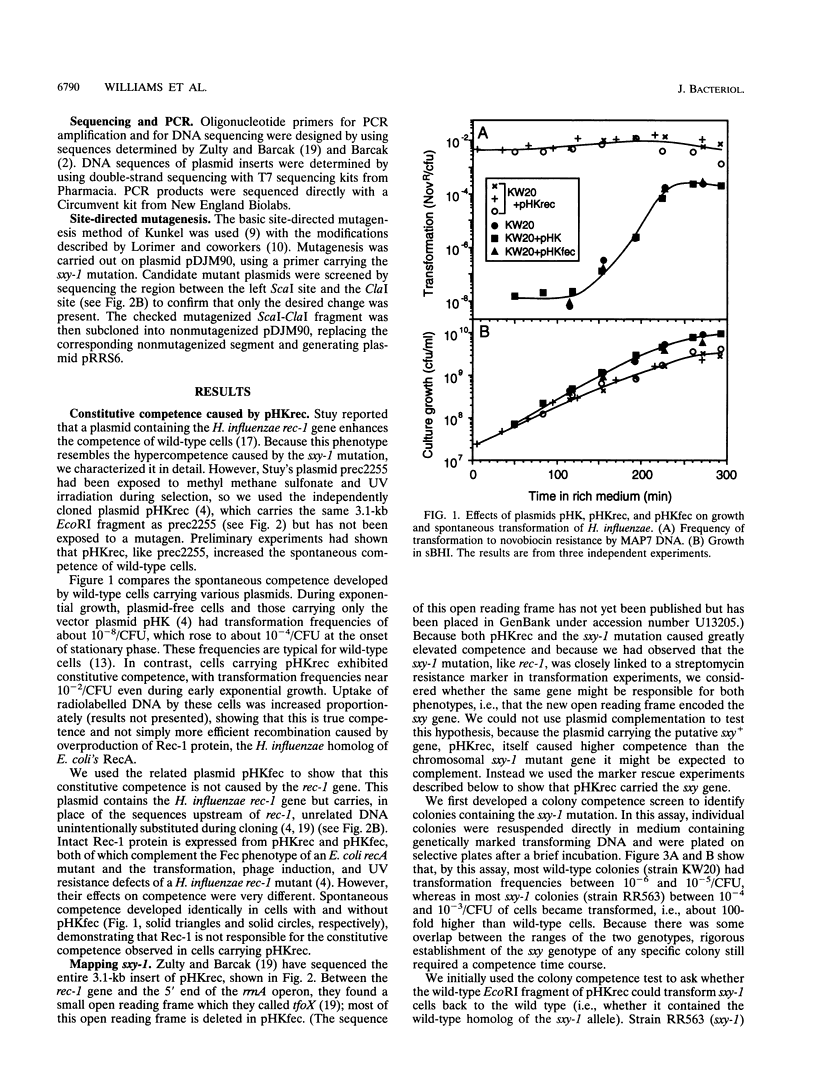
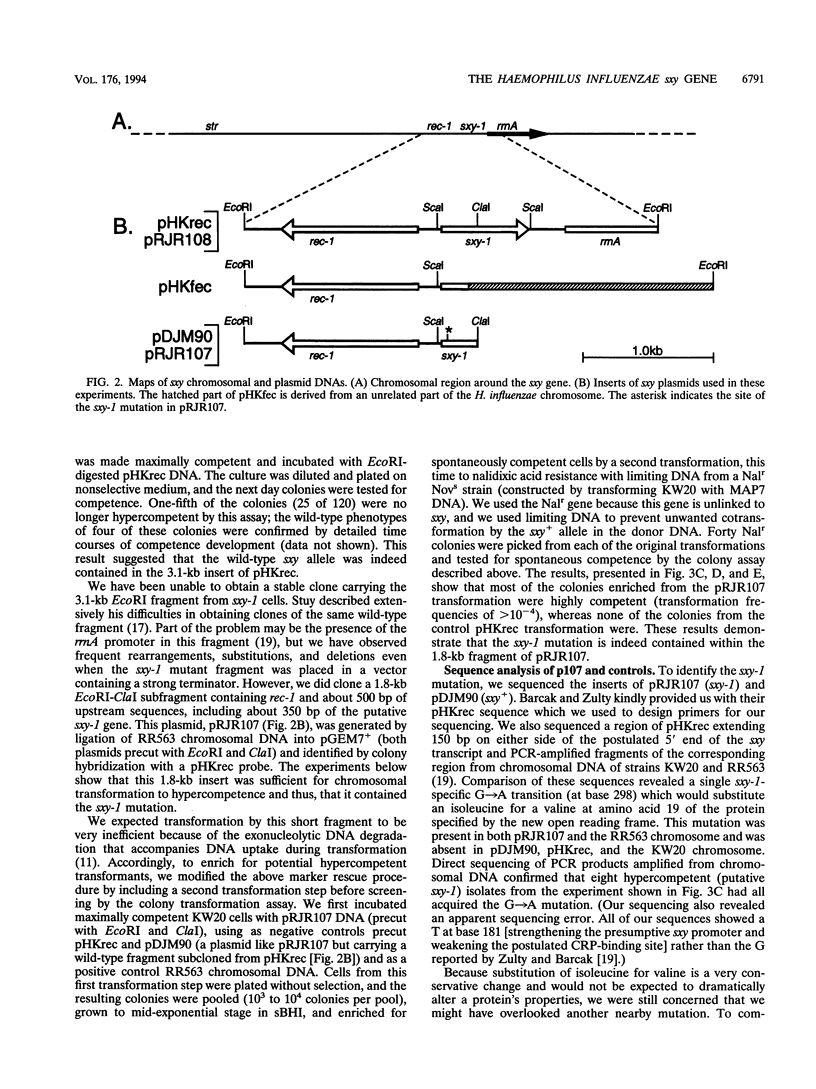
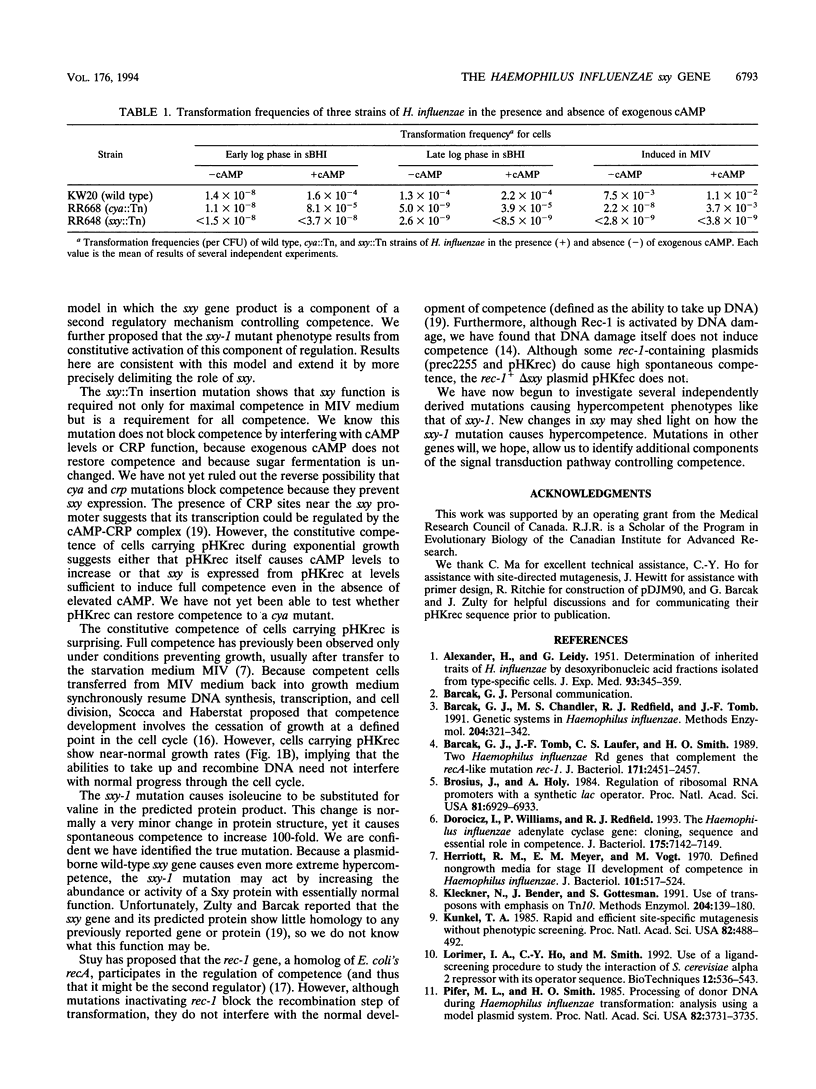
The sxy-1 mutation of Haemophilus influenzae causes a 100- to 1,000-fold increase in spontaneous natural competence. We have used mapping and sequencing to identify this mutation as a G-to-A transition in an open reading frame adjacent to the rec-1 locus. This mutation substitutes valine for isoleucine at amino acid 19 of the protein specified by this gene (now named sxy). A multicopy plasmid containing the wild-type sxy gene confers constitutive competence on wild-type cells. Cells carrying this plasmid exhibit, in all stages of growth, DNA uptake levels and transformation frequencies as high those normally seen only after full induction of competence by starvation; deletion of part of the sxy gene from the plasmid abolishes this effect. In contrast, a transposon insertion in sxy entirely prevents both DNA uptake and transformation, indicating that sxy encodes a function essential for competence. These findings suggest that sxy may act as a positive regulator of competence. However, because cells carrying the transposon-inactivated sxy::Tn allele grow slowly under conditions that do not induce competence, sxy may also have a role in noncompetent cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., LEIDY G. Determination of inherited traits of H. influenzae by desoxyribonucleic acid fractions isolated from type-specific cells. J Exp Med. 1951 Apr 1;93(4):345–359. doi: 10.1084/jem.93.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcak G. J., Chandler M. S., Redfield R. J., Tomb J. F. Genetic systems in Haemophilus influenzae. Methods Enzymol. 1991;204:321–342. doi: 10.1016/0076-6879(91)04016-h. [DOI] [PubMed] [Google Scholar]
- Barcak G. J., Tomb J. F., Laufer C. S., Smith H. O. Two Haemophilus influenzae Rd genes that complement the recA-like mutation rec-1. J Bacteriol. 1989 May;171(5):2451–2457. doi: 10.1128/jb.171.5.2451-2457.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Holy A. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorocicz I. R., Williams P. M., Redfield R. J. The Haemophilus influenzae adenylate cyclase gene: cloning, sequence, and essential role in competence. J Bacteriol. 1993 Nov;175(22):7142–7149. doi: 10.1128/jb.175.22.7142-7149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Bender J., Gottesman S. Uses of transposons with emphasis on Tn10. Methods Enzymol. 1991;204:139–180. doi: 10.1016/0076-6879(91)04009-d. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer I. A., Ho C. Y., Smith M. Use of a ligand-screening procedure to study the interaction of S. cerevisiae alpha 2 repressor with its operator sequence. Biotechniques. 1992 Apr;12(4):536–543. [PubMed] [Google Scholar]
- Pifer M. L., Smith H. O. Processing of donor DNA during Haemophilus influenzae transformation: analysis using a model plasmid system. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3731–3735. doi: 10.1073/pnas.82.11.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield R. J. Evolution of natural transformation: testing the DNA repair hypothesis in Bacillus subtilis and Haemophilus influenzae. Genetics. 1993 Apr;133(4):755–761. doi: 10.1093/genetics/133.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield R. J. Genes for breakfast: the have-your-cake-and-eat-it-too of bacterial transformation. J Hered. 1993 Sep-Oct;84(5):400–404. doi: 10.1093/oxfordjournals.jhered.a111361. [DOI] [PubMed] [Google Scholar]
- Redfield R. J. sxy-1, a Haemophilus influenzae mutation causing greatly enhanced spontaneous competence. J Bacteriol. 1991 Sep;173(18):5612–5618. doi: 10.1128/jb.173.18.5612-5618.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scocca J. J., Habersat M. Synchronous division and rates of macromolecular synthesis in Haemophilus influenzae competent for genetic transformation. J Bacteriol. 1978 Sep;135(3):961–967. doi: 10.1128/jb.135.3.961-967.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Cloning and characterization of the Haemophilus influenzae Rd rec-1+ gene. J Bacteriol. 1989 Aug;171(8):4395–4401. doi: 10.1128/jb.171.8.4395-4401.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Habersat M., Scocca J. J. Multiple regulatory events in the development of competence for genetic transformation in Haemophilus influenzae. J Bacteriol. 1975 Dec;124(3):1607–1609. doi: 10.1128/jb.124.3.1607-1609.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulty J. J., Barcak G. J. Structural organization, nucleotide sequence, and regulation of the Haemophilus influenzae rec-1+ gene. J Bacteriol. 1993 Nov;175(22):7269–7281. doi: 10.1128/jb.175.22.7269-7281.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]



