Abstract
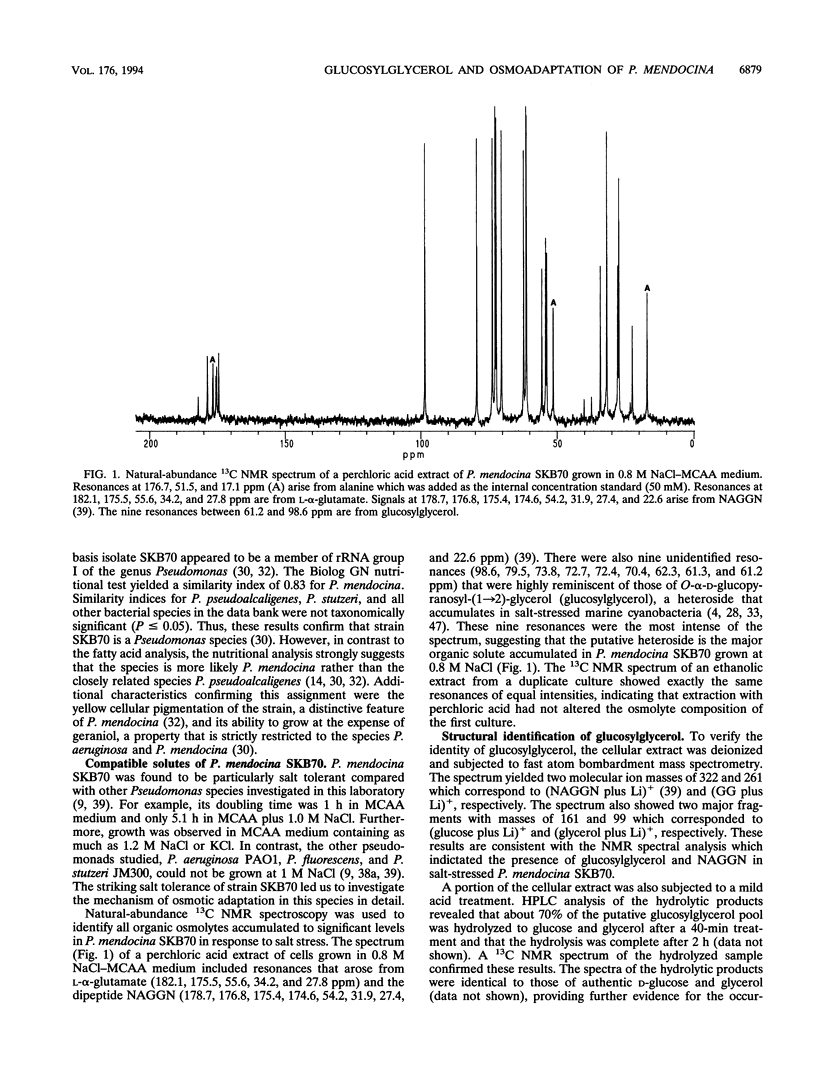
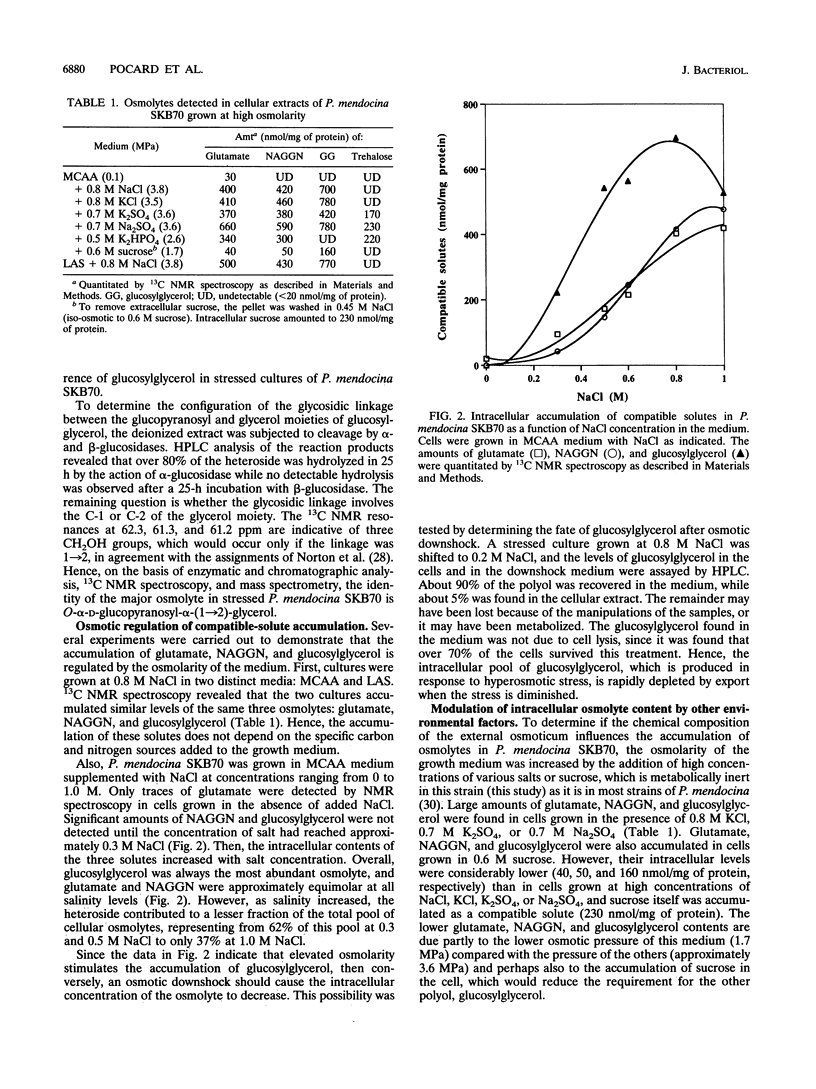
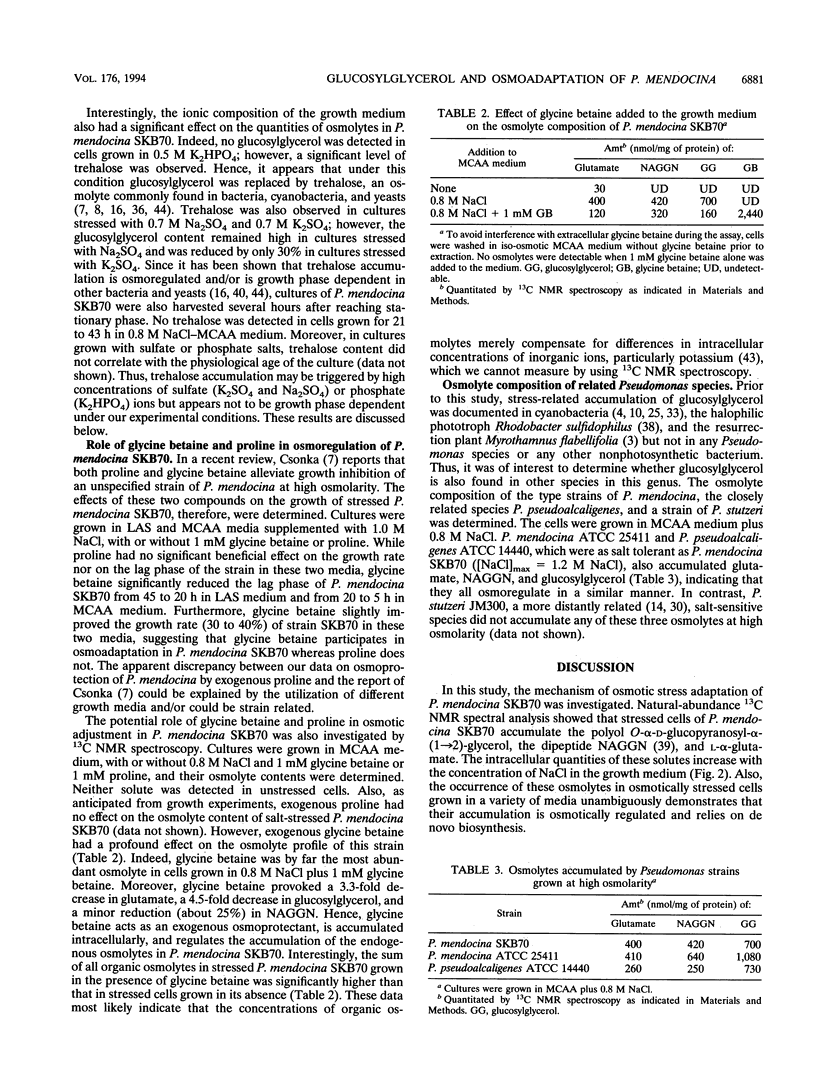
The mechanism of osmoadaptation in a salt-tolerant (1.2 M NaCl) bacterial isolate identified as Pseudomonas mendocina (N. J. Palleroni, M. Doudoroff, R. Y. Stanier, R. E. Solanes, and R. Mandel, J. Gen. Microbiol. 60:215-231, 1970) was investigated. In response to osmotic stress, this species accumulated a number of compatible solutes, the intracellular levels of which depended on both the osmolarity and the ionic composition of the growth medium. Glucosylglycerol [alpha-D-glucopyranosyl-alpha-(1-->2)-glycerol], N-acetylglutaminylglutamine amide, and L-alpha-glutamate were the major compatible solutes accumulated via de novo biosynthesis. Trehalose was also accumulated, but only in cells grown in the presence of high concentrations of sulfate or phosphate ions. Glycine betaine was accumulated only when supplied exogenously to cells grown at high osmolarity, and its accumulation caused a significant depletion of the intracellular pools of glucosylglycerol and glutamate. Glucosylglycerol was also found to accumulate in the type strains of P. mendocina and P. pseudoalcaligenes. This is the first report demonstrating the pivotal role of glucosylglycerol in osmoadaptation in a nonphotosynthetic microorganism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhrouf A., Jeddi M., Gauthier M. J. Survie du Salmonella paratyphi B et du Pseudomonas aeruginosa dans l'eau de mer après incubation ou lavage en présence d'osmolytes. Can J Microbiol. 1992 Jul;38(7):690–693. [PubMed] [Google Scholar]
- Borowitzka L. J., Demmerle S., Mackay M. A., Norton R. S. Carbon-13 nuclear magnetic resonance study of osmoregulation in a blue-green alga. Science. 1980 Nov 7;210(4470):650–651. doi: 10.1126/science.210.4470.650. [DOI] [PubMed] [Google Scholar]
- Carlson C. A., Pierson L. S., Rosen J. J., Ingraham J. L. Pseudomonas stutzeri and related species undergo natural transformation. J Bacteriol. 1983 Jan;153(1):93–99. doi: 10.1128/jb.153.1.93-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Hanson A. D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza-Ault M. R., Smith L. T., Smith G. M. Roles of N-acetylglutaminylglutamine amide and glycine betaine in adaptation of Pseudomonas aeruginosa to osmotic stress. Appl Environ Microbiol. 1993 Feb;59(2):473–478. doi: 10.1128/aem.59.2.473-478.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Perez A., Burg M. B. Renal medullary organic osmolytes. Physiol Rev. 1991 Oct;71(4):1081–1115. doi: 10.1152/physrev.1991.71.4.1081. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R., Klein W., Lange R., Rimmele M., Boos W. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J Bacteriol. 1991 Dec;173(24):7918–7924. doi: 10.1128/jb.173.24.7918-7924.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten U., Barrow K. D., King R. J. Floridoside, L-Isofloridoside, and D-Isofloridoside in the Red Alga Porphyra columbina (Seasonal and Osmotic Effects). Plant Physiol. 1993 Oct;103(2):485–491. doi: 10.1104/pp.103.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H., Thomson K. S., Thomson M., Jeblick W. Osmotic regulation: physiological significance of proteolytic and nonproteolytic activation of isofloridoside-phosphate synthase. Plant Physiol. 1979 Mar;63(3):455–459. doi: 10.1104/pp.63.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Norton R. S., MacKay M. A., Borowitzka L. J. The physical state of osmoregulatory solutes in unicellular algae. A natural-abundance carbon-13 nuclear-magnetic-resonance relaxation study. Biochem J. 1982 Mar 15;202(3):699–706. doi: 10.1042/bj2020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan D. J., O'Gara F. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol Rev. 1992 Dec;56(4):662–676. doi: 10.1128/mr.56.4.662-676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleroni N. J., Doudoroff M., Stanier R. Y., Solánes R. E., Mandel M. Taxonomy of the aerobic pseudomonads: the properties of the Pseudomonas stutzeri group. J Gen Microbiol. 1970 Feb;60(2):215–231. doi: 10.1099/00221287-60-2-215. [DOI] [PubMed] [Google Scholar]
- Smith L. T., Smith G. M., Madkour M. A. Osmoregulation in Agrobacterium tumefaciens: accumulation of a novel disaccharide is controlled by osmotic strength and glycine betaine. J Bacteriol. 1990 Dec;172(12):6849–6855. doi: 10.1128/jb.172.12.6849-6855.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Robertson D. E., Noll D., Gunsalus R. P., Roberts M. F. N epsilon-acetyl-beta-lysine: an osmolyte synthesized by methanogenic archaebacteria. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9083–9087. doi: 10.1073/pnas.87.23.9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M. Regulation of trehalose mobilization in fungi. Microbiol Rev. 1984 Mar;48(1):42–59. doi: 10.1128/mr.48.1.42-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. R., de Vos W. M., Harayama S., Zehnder A. J. Molecular mechanisms of genetic adaptation to xenobiotic compounds. Microbiol Rev. 1992 Dec;56(4):677–694. doi: 10.1128/mr.56.4.677-694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



