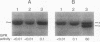Abstract
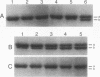
The sequence-specific protease (termed GPR) that degrades small, acid-soluble proteins (SASP) during germination of spores of Bacillus species is synthesized during sporulation as an inactive precursor termed P46. Approximately 2 h later in sporulation, P46 is converted proteolytically to a smaller form, termed P41, which is active in vitro, but which does not act significantly on SASP in vivo until spore germination is initiated. While it appears likely that P46-->P41 conversion is an autoprocessing event, the mechanisms regulating P46-->P41 conversion in vivo are not clear. In this work we found that P46-->P41 conversion in vitro was stimulated tremendously in an allosteric manner by pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) plus Ca2+ but not by Ca2+ in combination with a variety of DPA analogs. The processing reaction stimulated by Ca(2+)-DPA was seen at pH 5.1 but not at pH 6.2 or 7, and under these conditions P46-->P41 conversion exhibited a linear time course and was a first-order reaction, indicative of an intramolecular autoprocessing reaction. At pH 5.1, P46-->P41 conversion was stimulated markedly by very high ionic strength. At pHs from 5.1 to 6.6, P46-->P41 conversion also occurred when P46 was dehydrated to approximately 54% relative humidity. This processing was stimulated markedly when dehydration was carried out in the presence of DPA and NaCl but was greatly decreased when dehydration was to 10, 33, or 75% relative humidity. Since previous work has shown that P(46)-->P(41) processing in vivo takes place (i) after a fall in forespore pH to 6.3 to 6.9 and approximately in parallel with (ii) DPA accumulation by the forespore and (iii) dehydration of the forespore, out new finings in vitro suggest that these three changes may synergistically trigger P(46)-->P(41) autoprocessing in the developing forespore. Presumably the conditions in vivo during this authoprocessing preclude significant attack of the P(41) generated on its SASP substrates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bone S., Pethig R. Dielectric studies of the binding of water to lysozyme. J Mol Biol. 1982 May 25;157(3):571–575. doi: 10.1016/0022-2836(82)90477-6. [DOI] [PubMed] [Google Scholar]
- Careri G., Gratton E., Yang P. H., Rupley J. A. Correlation of IR spectroscopic, heat capacity, diamagnetic susceptibility and enzymatic measurements on lysozyme powder. Nature. 1980 Apr 10;284(5756):572–573. doi: 10.1038/284572a0. [DOI] [PubMed] [Google Scholar]
- Carrillo-Martinez Y., Setlow P. Properties of Bacillus subtilis small, acid-soluble spore proteins with changes in the sequence recognized by their specific protease. J Bacteriol. 1994 Sep;176(17):5357–5363. doi: 10.1128/jb.176.17.5357-5363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illades-Aguiar B., Setlow P. Studies of the processing of the protease which initiates degradation of small, acid-soluble proteins during germination of spores of Bacillus species. J Bacteriol. 1994 May;176(10):2788–2795. doi: 10.1128/jb.176.10.2788-2795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illades-Aguiar B., Setlow P. The zymogen of the protease that degrades small, acid-soluble proteins of spores of Bacillus species can rapidly autoprocess to the active enzyme in vitro. J Bacteriol. 1994 Sep;176(17):5571–5573. doi: 10.1128/jb.176.17.5571-5573.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loshon C. A., Setlow P. Bacillus megaterium spore protease: purification, radioimmunoassay, and analysis of antigen level and localization during growth, sporulation, and spore germination. J Bacteriol. 1982 Apr;150(1):303–311. doi: 10.1128/jb.150.1.303-311.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loshon C. A., Swerdlow B. M., Setlow P. Bacillus megaterium spore protease. Synthesis and processing of precursor forms during sporulation and germination. J Biol Chem. 1982 Sep 25;257(18):10838–10845. [PubMed] [Google Scholar]
- Magill N. G., Cowan A. E., Koppel D. E., Setlow P. The internal pH of the forespore compartment of Bacillus megaterium decreases by about 1 pH unit during sporulation. J Bacteriol. 1994 Apr;176(8):2252–2258. doi: 10.1128/jb.176.8.2252-2258.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. 18. Free amino acids in spores. J Biol Chem. 1970 Mar 10;245(5):1128–1136. [PubMed] [Google Scholar]
- POWELL J. F. Isolation of dipicolinic acid (pyridine-2:6-dicarboxylic acid) from spores of Bacillus megatherium. Biochem J. 1953 May;54(2):210–211. doi: 10.1042/bj0540210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn R. O., Hosszu H. L. Influence of relative humidity on the photochemistry of DNA films. Biochim Biophys Acta. 1969 Sep 17;190(1):126–131. doi: 10.1016/0005-2787(69)90161-0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Salas J. L., Setlow P. Proteolytic processing of the protease which initiates degradation of small, acid-soluble proteins during germination of Bacillus subtilis spores. J Bacteriol. 1993 May;175(9):2568–2577. doi: 10.1128/jb.175.9.2568-2577.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Measurements of the pH within dormant and germinated bacterial spores. Proc Natl Acad Sci U S A. 1980 May;77(5):2474–2476. doi: 10.1073/pnas.77.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. I will survive: protecting and repairing spore DNA. J Bacteriol. 1992 May;174(9):2737–2741. doi: 10.1128/jb.174.9.2737-2741.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Mechanisms which contribute to the long-term survival of spores of Bacillus species. Soc Appl Bacteriol Symp Ser. 1994;23:49S–60S. doi: 10.1111/j.1365-2672.1994.tb04357.x. [DOI] [PubMed] [Google Scholar]
- Setlow P. Small, acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function, and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- Singh R. P., Setlow B., Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977 Jun;130(3):1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. X., Setlow P. Cloning, nucleotide sequence, and expression of the Bacillus subtilis ans operon, which codes for L-asparaginase and L-aspartase. J Bacteriol. 1991 Jun;173(12):3831–3845. doi: 10.1128/jb.173.12.3831-3845.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. D., Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis gpr gene, which codes for the protease that initiates degradation of small, acid-soluble proteins during spore germination. J Bacteriol. 1991 Jan;173(1):291–300. doi: 10.1128/jb.173.1.291-300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]