Abstract
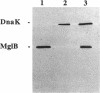
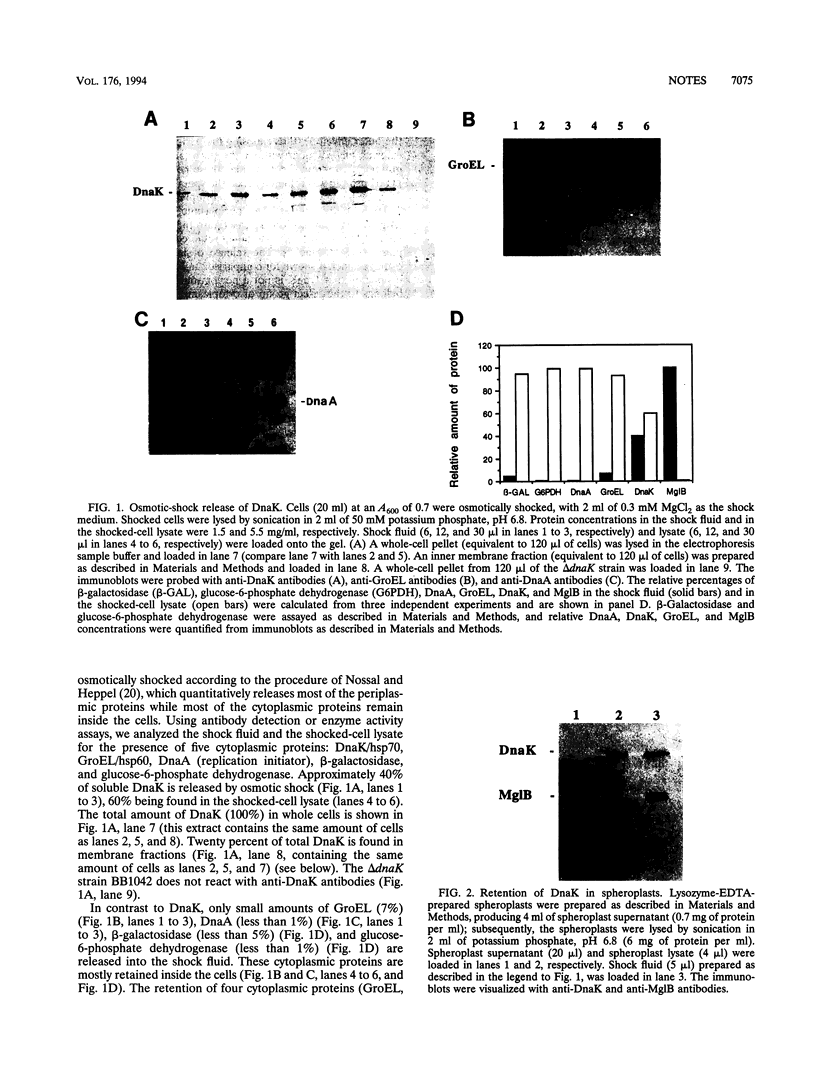
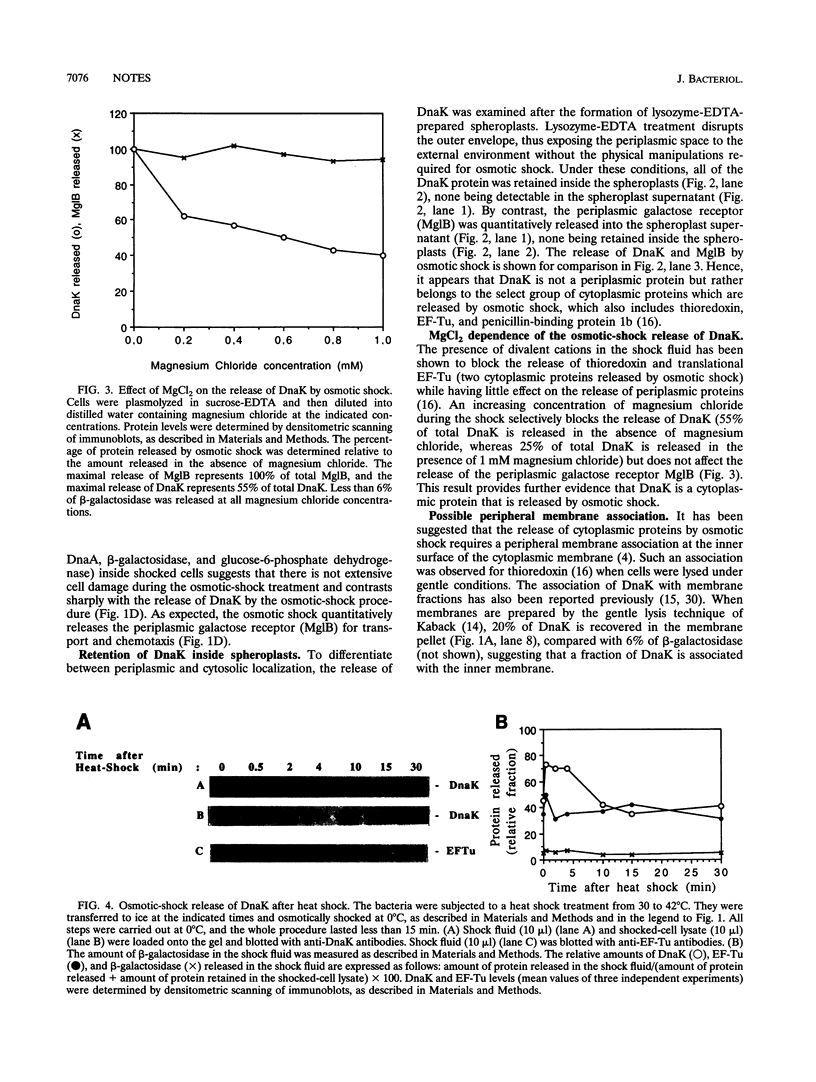
The chaperone DnaK can be released (up to 40%) by osmotic shock, a procedure which is known to release the periplasmic proteins and a select group of cytoplasmic proteins (including thioredoxin and elongation factor Tu) possibly associated with the inner face of the inner membrane. As distinct from periplasmic proteins, DnaK is retained within spheroplasts prepared with lysozyme and EDTA. The ability to isolate DnaK with a membrane fraction prepared under gentle lysis conditions supports a peripheral association between DnaK and the cytoplasmic membrane. Furthermore, heat shock transiently increases the localization of DnaK in the osmotic-shock-sensitive compartment of the cytoplasm. We conclude that DnaK belongs to the select group of cytoplasmic proteins released by osmotic shock, which are possibly located at Bayer adhesion sites, where the inner and outer membranes are contiguous.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Bayer M. H., Lunn C. A., Pigiet V. Association of thioredoxin with the inner membrane and adhesion sites in Escherichia coli. J Bacteriol. 1987 Jun;169(6):2659–2666. doi: 10.1128/jb.169.6.2659-2666.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. H., Costello G. P., Bayer M. E. Isolation and partial characterization of membrane vesicles carrying markers of the membrane adhesion sites. J Bacteriol. 1982 Feb;149(2):758–767. doi: 10.1128/jb.149.2.758-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham I. R. Periplasmic enzymes in gram-negative bacteria. Int J Biochem. 1979;10(11):877–883. doi: 10.1016/0020-711x(79)90117-4. [DOI] [PubMed] [Google Scholar]
- Bukau B., Reilly P., McCarty J., Walker G. C. Immunogold localization of the DnaK heat shock protein in Escherichia coli cells. J Gen Microbiol. 1993 Jan;139(1):95–99. doi: 10.1099/00221287-139-1-95. [DOI] [PubMed] [Google Scholar]
- Bukau B., Walker G. C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicate roles for heat shock protein in normal metabolism. J Bacteriol. 1989 May;171(5):2337–2346. doi: 10.1128/jb.171.5.2337-2346.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G., Levisohn S. R. Glucose and gluconate metabolism in an Escherichia coli mutant lacking phosphoglucose isomerase. J Bacteriol. 1967 May;93(5):1571–1578. doi: 10.1128/jb.93.5.1571-1578.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P. A new bacterial gene (groPC) which affects lambda DNA replication. Mol Gen Genet. 1977 Feb 28;151(1):35–39. doi: 10.1007/BF00446910. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979 Apr 15;129(3):375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- Ishidate K., Creeger E. S., Zrike J., Deb S., Glauner B., MacAlister T. J., Rothfield L. I. Isolation of differentiated membrane domains from Escherichia coli and Salmonella typhimurium, including a fraction containing attachment sites between the inner and outer membranes and the murein skeleton of the cell envelope. J Biol Chem. 1986 Jan 5;261(1):428–443. [PubMed] [Google Scholar]
- Jacobson G. R., Rosenbusch J. P. Abundance and membrane association of elongation factor Tu in E. coli. Nature. 1976 May 6;261(5555):23–26. doi: 10.1038/261023a0. [DOI] [PubMed] [Google Scholar]
- Kostyal D. A., Farrell M., McCabe A., Mei Z., Firshein W. Replication of an RK2 miniplasmid derivative in vitro by a DNA/membrane complex extracted from Escherichia coli: involvement of the dnaA but not dnaK host proteins and association of these and plasmid-encoded proteins with the inner membrane. Plasmid. 1989 May;21(3):226–237. doi: 10.1016/0147-619x(89)90046-2. [DOI] [PubMed] [Google Scholar]
- Lunn C. A., Pigiet V. P. Localization of thioredoxin from Escherichia coli in an osmotically sensitive compartment. J Biol Chem. 1982 Oct 10;257(19):11424–11430. [PubMed] [Google Scholar]
- Nilsson B., Berman-Marks C., Kuntz I. D., Anderson S. Secretion incompetence of bovine pancreatic trypsin inhibitor expressed in Escherichia coli. J Biol Chem. 1991 Feb 15;266(5):2970–2977. [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Richarme G. Associative properties of the Escherichia coli galactose-binding protein and maltose-binding protein. Biochim Biophys Acta. 1983 Oct 17;748(1):99–108. doi: 10.1016/0167-4838(83)90032-8. [DOI] [PubMed] [Google Scholar]
- Richarme G., Kohiyama M. Specificity of the Escherichia coli chaperone DnaK (70-kDa heat shock protein) for hydrophobic amino acids. J Biol Chem. 1993 Nov 15;268(32):24074–24077. [PubMed] [Google Scholar]
- Russel M., Model P. Thioredoxin is required for filamentous phage assembly. Proc Natl Acad Sci U S A. 1985 Jan;82(1):29–33. doi: 10.1073/pnas.82.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Bieker K. L., Ottemann K. M., Silhavy T. J., Beckwith J. One of three transmembrane stretches is sufficient for the functioning of the SecE protein, a membrane component of the E. coli secretion machinery. EMBO J. 1991 Jul;10(7):1749–1757. doi: 10.1002/j.1460-2075.1991.tb07699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Zhou Y., Wild J., Adler J., Gross C. A. DnaK, DnaJ, and GrpE are required for flagellum synthesis in Escherichia coli. J Bacteriol. 1992 Oct;174(19):6256–6263. doi: 10.1128/jb.174.19.6256-6263.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Georgopoulos C., Ang D. The essential Escherichia coli msgB gene, a multicopy suppressor of a temperature-sensitive allele of the heat shock gene grpE, is identical to dapE. J Bacteriol. 1992 Aug;174(16):5258–5264. doi: 10.1128/jb.174.16.5258-5264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M., Ang D., Georgopoulos C. The grpE protein of Escherichia coli. Purification and properties. J Biol Chem. 1987 Dec 25;262(36):17437–17442. [PubMed] [Google Scholar]
- Zylicz M., Georgopoulos C. Purification and properties of the Escherichia coli dnaK replication protein. J Biol Chem. 1984 Jul 25;259(14):8820–8825. [PubMed] [Google Scholar]
- Zylicz M., Nieradko J., Taylor K. Escherichia coli dnaJ- and dnaK-gene products: synthesis in minicells and membrane-affinity. Biochem Biophys Res Commun. 1983 Jan 14;110(1):176–180. doi: 10.1016/0006-291x(83)91276-7. [DOI] [PubMed] [Google Scholar]