Abstract
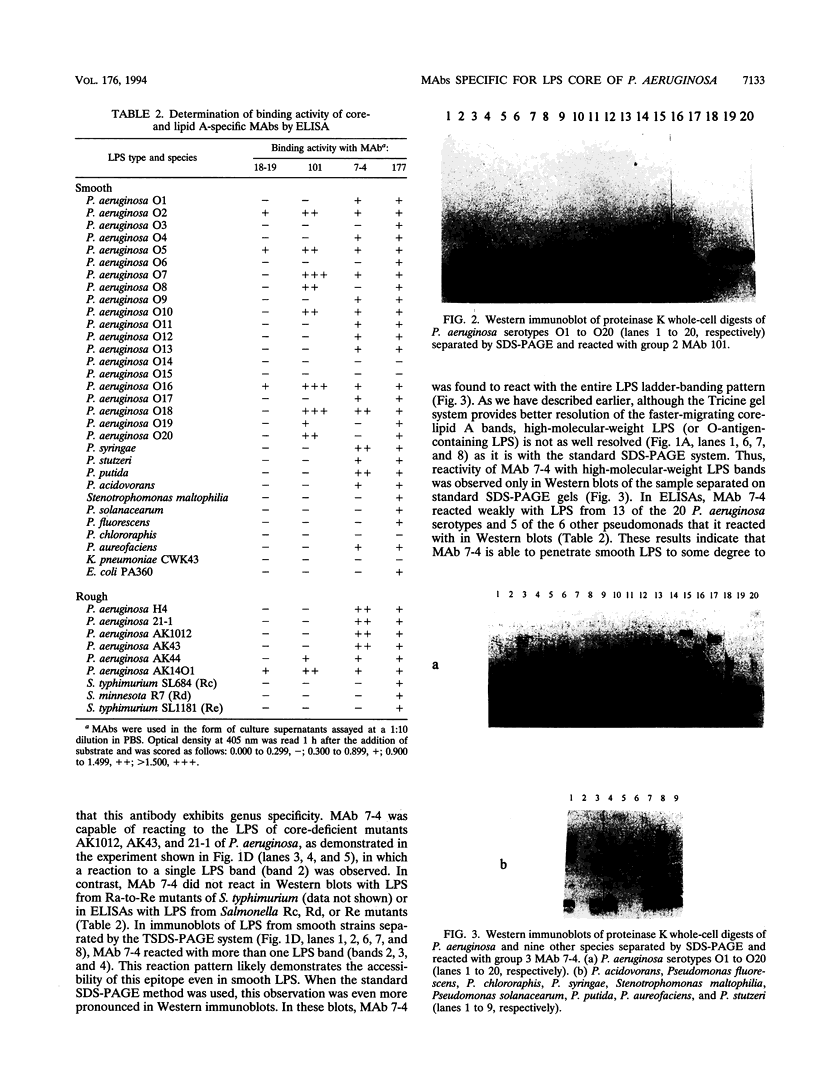
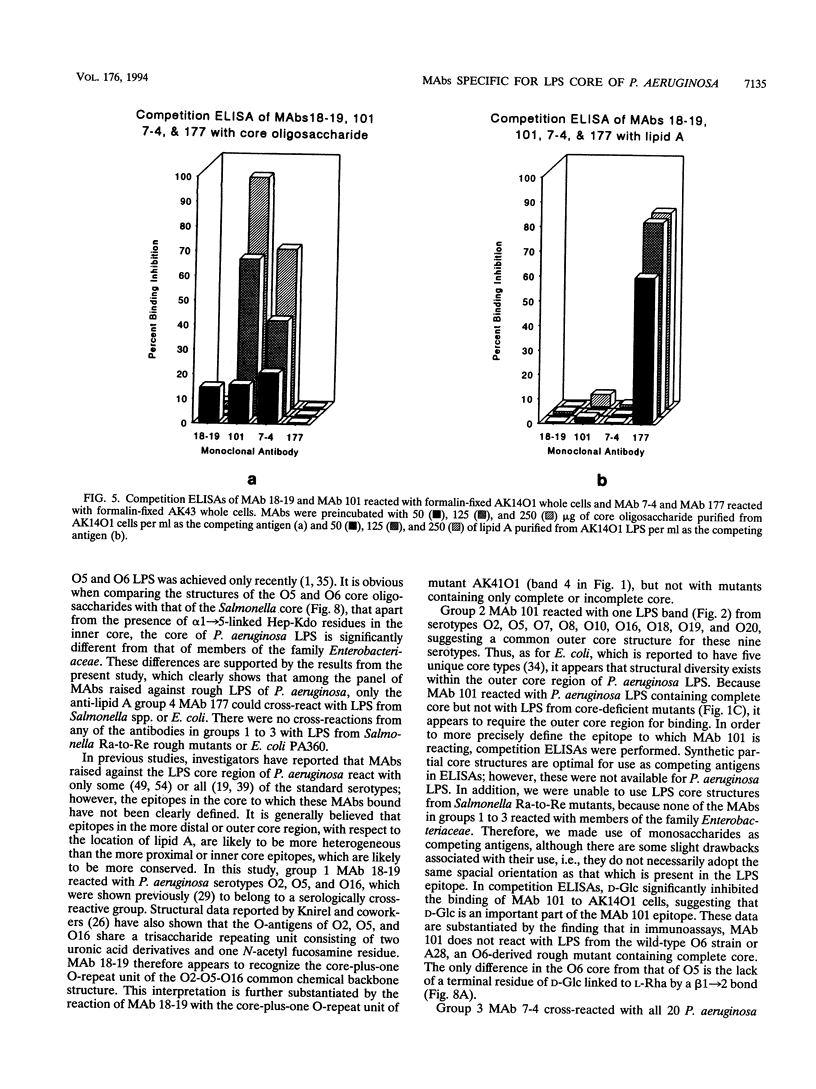
In order to examine the immunochemistry of the core-lipid A region of Pseudomonas aeruginosa lipopolysaccharide (LPS), monoclonal antibodies (MAbs) specific for this region were produced in mice. Immunogen was prepared by coating a rough mutant of P. aeruginosa with column-purified core oligosaccharide fractions in order to enhance the immune response to the LPS core-lipid A region. Fourteen hybridoma clones were isolated, characterized, and further divided into three groups on the basis of their reactivities to rough LPS antigens in both enzyme-linked immunosorbent assays and Western immunoblots. In addition, another MAb, 18-19, designated group 1, was included in this study for defining core-lipid A epitopes. MAb 18-19 recognizes the LPS core-plus-one O-repeat unit of the serologically cross-reactive P. aeruginosa O2, O5, and O16. Group 2 MAbs are specific for the LPS outer core region and reacted with P. aeruginosa O2, O5, O7, O8, O10, O16, O18, O19, and O20, suggesting that these serotypes share a common outer core type. Group 3 MAbs recognize the inner core region and reacted with all 20 P. aeruginosa serotypes as well as with other Pseudomonas species, revealing the conserved nature of this region. Group 4 MAbs are specific for lipid A and reacted with all gram-negative organisms tested. Immunoassays using these MAbs and well-defined rough mutants, in addition to the recently determined P. aeruginosa core structures, have allowed us to precisely define immunodominant epitopes within the LPS core region.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bantroch S., Bühler T., Lam J. S. Appropriate coating methods and other conditions for enzyme-linked immunosorbent assay of smooth, rough, and neutral lipopolysaccharides of Pseudomonas aeruginosa. Clin Diagn Lab Immunol. 1994 Jan;1(1):55–62. doi: 10.1128/cdli.1.1.55-62.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D., Kropinski A. M. Effect of lipopolysaccharide mutations and temperature on plasmid transformation efficiency in Pseudomonas aeruginosa. Can J Microbiol. 1986 May;32(5):436–438. doi: 10.1139/m86-082. [DOI] [PubMed] [Google Scholar]
- Bogard W. C., Jr, Dunn D. L., Abernethy K., Kilgarriff C., Kung P. C. Isolation and characterization of murine monoclonal antibodies specific for gram-negative bacterial lipopolysaccharide: association of cross-genus reactivity with lipid A specificity. Infect Immun. 1987 Apr;55(4):899–908. doi: 10.1128/iai.55.4.899-908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen L., Coleman W. G., Jr Cloning and characterization of the Escherichia coli K-12 rfa-2 (rfaC) gene, a gene required for lipopolysaccharide inner core synthesis. J Bacteriol. 1993 May;175(9):2534–2540. doi: 10.1128/jb.175.9.2534-2540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Pitt T. L., Fürer E., Germanier R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect Immun. 1984 May;44(2):508–513. doi: 10.1128/iai.44.2.508-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta T., de Kievit T. R., Masoud H., Altman E., Richards J. C., Sadovskaya I., Speert D. P., Lam J. S. Characterization of lipopolysaccharide-deficient mutants of Pseudomonas aeruginosa derived from serotypes O3, O5, and O6. Infect Immun. 1994 Mar;62(3):809–817. doi: 10.1128/iai.62.3.809-817.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Padova F. E., Brade H., Barclay G. R., Poxton I. R., Liehl E., Schuetze E., Kocher H. P., Ramsay G., Schreier M. H., McClelland D. B. A broadly cross-protective monoclonal antibody binding to Escherichia coli and Salmonella lipopolysaccharides. Infect Immun. 1993 Sep;61(9):3863–3872. doi: 10.1128/iai.61.9.3863-3872.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry D. T., Symes K. C., Gray G. W., Wilkinson S. G. Studies of polysaccharide fractions from the lipopolysaccharide of Pseudomonas aeruginosa N.C.T.C. 1999. Biochem J. 1975 Jul;149(1):93–106. doi: 10.1042/bj1490093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Bogard W. C., Jr, Cerra F. B. Efficacy of type-specific and cross-reactive murine monoclonal antibodies directed against endotoxin during experimental sepsis. Surgery. 1985 Aug;98(2):283–290. [PubMed] [Google Scholar]
- Dunn D. L., Ewald D. C., Chandan N., Cerra F. B. Immunotherapy of gram-negative bacterial sepsis. A single murine monoclonal antibody provides cross-genera protection. Arch Surg. 1986 Jan;121(1):58–62. doi: 10.1001/archsurg.1986.01400010064008. [DOI] [PubMed] [Google Scholar]
- Emori T. G., Gaynes R. P. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993 Oct;6(4):428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara Y., Bogard W. C., Lei M. G., Daddona P. E., Morrison D. C. Monoclonal anti-lipid A IgM antibodies HA-1A and E-5 recognize distinct epitopes on lipopolysaccharide and lipid A. J Infect Dis. 1993 Dec;168(6):1429–1435. doi: 10.1093/infdis/168.6.1429. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. F., Kropinski A. M. Isolation and characterization of a bacteriophage specific for the lipopolysaccharide of rough derivatives of Pseudomonas aeruginosa strain PAO. J Virol. 1981 May;38(2):529–538. doi: 10.1128/jvi.38.2.529-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastowsky M., Gutberlet T., Bradaczek H. Molecular modelling of the three-dimensional structure and conformational flexibility of bacterial lipopolysaccharide. J Bacteriol. 1992 Jul;174(14):4798–4806. doi: 10.1128/jb.174.14.4798-4806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi M., Huntenburg C. C., Bubbers J. E. Lipopolysaccharide epitope specificity and binding cross reactivity of the human IgM anti-lipid A monoclonal antibody SdJ5-1.17.15. Mol Immunol. 1993 Jul;30(10):895–902. doi: 10.1016/0161-5890(93)90013-2. [DOI] [PubMed] [Google Scholar]
- Knirel YuA, Vinogradov E. V., Kocharova N. A., Paramonov N. A., Kochetkov N. K., Dmitriev B. A., Stanislavsky E. S., Lányi B. The structure of O-specific polysaccharides and serological classification of Pseudomonas aeruginosa (a review). Acta Microbiol Hung. 1988;35(1):3–24. [PubMed] [Google Scholar]
- Kropinski A. M., Chan L. C., Milazzo F. H. The extraction and analysis of lipopolysaccharides from Pseudomonas aeruginosa strain PAO, and three rough mutants. Can J Microbiol. 1979 Mar;25(3):390–398. doi: 10.1139/m79-060. [DOI] [PubMed] [Google Scholar]
- Lam J. S., Handelsman M. Y., Chivers T. R., MacDonald L. A. Monoclonal antibodies as probes to examine serotype-specific and cross-reactive epitopes of lipopolysaccharides from serotypes O2, O5, and O16 of Pseudomonas aeruginosa. J Bacteriol. 1992 Apr;174(7):2178–2184. doi: 10.1128/jb.174.7.2178-2184.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. S., MacDonald L. A., Lam M. Y., Duchesne L. G., Southam G. G. Production and characterization of monoclonal antibodies against serotype strains of Pseudomonas aeruginosa. Infect Immun. 1987 May;55(5):1051–1057. doi: 10.1128/iai.55.5.1051-1057.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesse A. J., Campagnari A. A., Bittner W. E., Apicella M. A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990 Jan 24;126(1):109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Wang S. Three new major somatic antigens of Pseudomonas aeruginosa. J Clin Microbiol. 1990 May;28(5):922–925. doi: 10.1128/jcm.28.5.922-925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoud H., Altman E., Richards J. C., Lam J. S. General strategy for structural analysis of the oligosaccharide region of lipooligosaccharides. Structure of the oligosaccharide component of Pseudomonas aeruginosa IATS serotype 06 mutant R5 rough-type lipopolysaccharide. Biochemistry. 1994 Sep 6;33(35):10568–10578. doi: 10.1021/bi00201a002. [DOI] [PubMed] [Google Scholar]
- Miner K. M., Manyak C. L., Williams E., Jackson J., Jewell M., Gammon M. T., Ehrenfreund C., Hayes E., Callahan L. T., 3rd, Zweerink H. Characterization of murine monoclonal antibodies to Escherichia coli J5. Infect Immun. 1986 Apr;52(1):56–62. doi: 10.1128/iai.52.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Crockford G., Bogard W. C., Jr, Hancock R. E. Monoclonal antibodies specific for Escherichia coli J5 lipopolysaccharide: cross-reaction with other gram-negative bacterial species. Infect Immun. 1984 Sep;45(3):631–636. doi: 10.1128/iai.45.3.631-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles M. J., Niswander C. A. Mouse monoclonal antibodies reactive with J5 lipopolysaccharide exhibit extensive serological cross-reactivity with a variety of gram-negative bacteria. Infect Immun. 1984 Dec;46(3):677–681. doi: 10.1128/iai.46.3.677-681.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. W., Barclay G. R., Micklem L. R., Poxton I. R., Govan J. R. Production and characterisation of mouse monoclonal antibodies reactive with the lipopolysaccharide core of Pseudomonas aeruginosa. J Med Microbiol. 1992 May;36(5):358–365. doi: 10.1099/00222615-36-5-358. [DOI] [PubMed] [Google Scholar]
- Pollack M., Chia J. K., Koles N. L., Miller M., Guelde G. Specificity and cross-reactivity of monoclonal antibodies reactive with the core and lipid A regions of bacterial lipopolysaccharide. J Infect Dis. 1989 Feb;159(2):168–188. doi: 10.1093/infdis/159.2.168. [DOI] [PubMed] [Google Scholar]
- Pollack M., Raubitschek A. A., Larrick J. W. Human monoclonal antibodies that recognize conserved epitopes in the core-lipid A region of lipopolysaccharides. J Clin Invest. 1987 May;79(5):1421–1430. doi: 10.1172/JCI112970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter A. A., Loutit J. S. Exonuclease activity from Pseudomonas aeruginosa which is missing in phenotypically restrictionless mutants. J Bacteriol. 1982 Sep;151(3):1204–1209. doi: 10.1128/jb.151.3.1204-1209.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest B. P., Brinson D. N., Schroeder D. A., Dunn D. L. Treatment of experimental gram-negative bacterial sepsis with murine monoclonal antibodies directed against lipopolysaccharide. Surgery. 1989 Aug;106(2):147–155. [PubMed] [Google Scholar]
- Rivera M., Bryan L. E., Hancock R. E., McGroarty E. J. Heterogeneity of lipopolysaccharides from Pseudomonas aeruginosa: analysis of lipopolysaccharide chain length. J Bacteriol. 1988 Feb;170(2):512–521. doi: 10.1128/jb.170.2.512-521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M., Chivers T. R., Lam J. S., McGroarty E. J. Common antigen lipopolysaccharide from Pseudomonas aeruginosa AK1401 as a receptor for bacteriophage A7. J Bacteriol. 1992 Apr;174(7):2407–2411. doi: 10.1128/jb.174.7.2407-2411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M., McGroarty E. J. Analysis of a common-antigen lipopolysaccharide from Pseudomonas aeruginosa. J Bacteriol. 1989 Apr;171(4):2244–2248. doi: 10.1128/jb.171.4.2244-2248.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe P. S., Meadow P. M. Structure of the Core oligosaccharide from the lipopolysaccharide of Pseudomonas aeruginosa PAC1R and its defective mutants. Eur J Biochem. 1983 May 2;132(2):329–337. doi: 10.1111/j.1432-1033.1983.tb07366.x. [DOI] [PubMed] [Google Scholar]
- Sadoff J. C., Wright D. C., Futrovsky S., Sidberry H., Collins H., Kaufmann B. Characterization of mouse monoclonal antibodies directed against Pseudomonas aeruginosa lipopolysaccharides. Antibiot Chemother (1971) 1985;36:134–146. doi: 10.1159/000410478. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L. Pseudomonas aeruginosa: biology, mechanisms of virulence, epidemiology. J Pediatr. 1986 May;108(5 Pt 2):800–805. doi: 10.1016/s0022-3476(86)80748-x. [DOI] [PubMed] [Google Scholar]
- Yokota S., Ochi H., Ohtsuka H., Kato M., Noguchi H. Heterogeneity of the L-rhamnose residue in the outer core of Pseudomonas aeruginosa lipopolysaccharide, characterized by using human monoclonal antibodies. Infect Immun. 1989 Jun;57(6):1691–1696. doi: 10.1128/iai.57.6.1691-1696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S., Terashima M., Chiba J., Noguchi H. Variable cross-reactivity of Pseudomonas aeruginosa lipopolysaccharide-code-specific monoclonal antibodies and its possible relationship with serotype. J Gen Microbiol. 1992 Feb;138(2):289–296. doi: 10.1099/00221287-138-2-289. [DOI] [PubMed] [Google Scholar]
- Young L. S., Gascon R., Alam S., Bermudez L. E. Monoclonal antibodies for treatment of gram-negative infections. Rev Infect Dis. 1989 Nov-Dec;11 (Suppl 7):S1564–S1571. doi: 10.1093/clinids/11.supplement_7.s1564. [DOI] [PubMed] [Google Scholar]








