Abstract
Passage of the mouse-adapted rabies virus strain CVS-24 (where CVS is challenge virus standard) in BHK cells results in the rapid selection of a dominant variant designated CVS-B2c that differs genotypically and phenotypically from the dominant variant CVS-N2c present in mouse-brain- or neuroblastoma-cell-passaged CVS-24. The glycoprotein of CVS-B2c has 10 amino acid substitutions compared with that of CVS-N2c. Because CVS-B2c can be reproducibly selected in BHK cells, it is likely to be a conserved minor subpopulation of CVS-24. CVS-N2c is more neurotropic in vitro and in vivo than CVS-B2c, which replicates more readily in nonneuronal cells in vitro and in vivo. These characteristics appear to be relevant to the pathogenicity of the two variants. CVS-N2c is more pathogenic for adult mice than CVS-B2c. In contrast, CVS-B2c is more pathogenic for neonatal mice. These differences in pathogenicity are reflected in the selection pattern when mixtures of CVS-N2c and CVS-B2c were used to infect neonatal and adult mice. Although CVS-N2c was highly selected in adult mice, no selection for either variant was seen in neonates, suggesting that certain aspects of development, such as maturation of the nervous and immune systems, may contribute to the selection process. We speculate that the existence of different variants within a rabies virus strain may facilitate the virus in overcoming barriers to its spread, both within the host and between species.
By far the predominant target of rabies virus infection in vivo is the neuron (1). Nevertheless, it has been shown that rabies virus can infect myocytes before the virus infection reaches neurons in the spinal cord or dorsal root ganglia (1, 2). In addition, high titers of rabies virus can be produced in mucogenic acinary cells of the salivary glands during the final phase of infection. In vitro experimentation has shown that street rabies virus can infect macrophages (3) and fixed rabies virus strains can infect murine lymphocytes and human lymphoblast T cells (4). However, it is not clear whether virus replication in nonneuronal tissue, with the exception of the salivary glands, actually represents an integral part of the pathogenesis of rabies nor it is known whether a single virus population or different subpopulations of a particular strain are responsible for the infection of neuronal and nonneuronal cells. In this context, it has been shown, in mice persistently infected with lymphocytic choriomeningitis virus (LCMV), that different variants of the same LCMV strain are associated with infection of neuronal cells, liver cells, or spleen cells (5, 6). Because of the high mutation rates resulting from the lack of proofreading by RNA polymerases RNA virus strains exist as quasispecies (7). It has been demonstrated that in a constant environment quasispecies contain a stable dominant virus species within a pool of viruses with related but often extremely heterogeneous genomes (7–9). Such complexity endows RNA viruses with an enormous capacity to adapt to changing host environments (10) and may explain the emergence of new viral pathogens, such as the silver-haired bat rabies virus, the etiological agent of most of the recent human rabies cases in North America (11). In this study we provide evidence that a particular rabies virus strain consists of variants with different biological properties and that changes in the host environment rapidly results in shifts in the dominant variant.
MATERIALS AND METHODS
Virus.
The suckling-mouse-brain-adapted CVS-24 rabies virus strain (where CVS is challenge virus standard) that has been passaged and maintained in suckling mice for several decades and has never been passaged in tissue culture was used for the studies below. Variants were derived by passaging of CVS-24 twice at low multiplicity (multiplicity of infectivity, 0.1) in mouse neuroblastoma cells (clone NA) or BHK-21 cells. The virus obtained from the second NA or BHK cell passage was subjected to two consecutive limit dilution purifications as described below. This approach was used because CVS-24 does not form plaques. Monolayers of NA or BHK-21 cells in 24-well plates were infected with 200 μl of NA or BHK cell-passaged virus at 1:10 dilutions for 1 hr at 37°C. The virus inoculum was then removed and the cultures were replenished with 1 ml of culture medium and incubated for 72 hr at 34°C. The tissue culture supernatants then were collected, and the cells were fixed with acetone and subjected to fluorescent staining with rabies virus-specific antibodies as described (12). Tissue culture supernatants obtained from rabies virus-positive cultures infected with the highest virus dilution were subjected to a second limit dilution purification procedure.
Preparation of Virus Stocks and Titration of Virus.
CVS-24 and the cloned variant CVS-N2c virus were propagated in suckling mice because low titers were obtained in tissue culture. One-day-old mice were infected intracranially with 103 focus-forming units (FFU) of CVS-24 or CVS-N2c. Seventy-two hours after infection, the mice were euthanized with CO2, brains were harvested, and a 20% brain suspension in PBS was prepared. The cloned rabies virus variant CVS-B2c was propagated in BHK-21 cells. Monolayer cultures of BHK-21 cells were grown at 37°C in Eagle’s minimum essential medium and infected with CVS-B2c at a multiplicity of infectivity of 0.1.
To determine virus titers, monolayers of mouse neuroblastoma cells or BHK-21 cells in 96-well plates were infected with 50 μl of stock virus at serial 1:10 dilutions and incubated for 1 hr to allow for virus adsorption. The virus inoculum was then removed, and the cultures were replenished with 100 μl of culture medium and then incubated at 34°C. Forty-eight hours after infection, the cells were fixed in 80% acetone and subjected to a fluorescent staining technique with rabies nucleoprotein (N)-specific antibody (12). Foci were counted by using a fluorescent microscope. All titrations were carried out in triplicate.
Virus Infection of Mice.
Six- to 8-week-old female Swiss Webster mice and pregnant mice at 14 days gestation were purchased from Taconic Farms. Mice were maintained under pathogen-free conditions and adult mice were used at 8–10 weeks of age or their offspring at the day after birth. Groups of ten 8- to 10-week-old mice or newborn mice were infected intracranially, intramuscularly, or intradermally under anesthesia with 10 μl of serial 1:10 dilutions of CVS-24, CVS-N2c, or CVS-B2c. After infection, the mice were observed daily for at least 4 weeks and, based on the percentage of surviving animals in each group, the LD50 value was calculated as described (13).
Extraction of RNA, Reverse Transcriptase–PCR (RT-PCR), Southern Blot Analysis, Sequencing of the Rabies Virus G Protein Gene, and Restriction Enzyme Analysis.
Total RNA was isolated from virus-infected mouse tissues or tissue cultures according to the manufacturer’s manual for the RNAzol B method (Biotecx Laboratories, Houston). Virus genomic RNA was isolated from rabies virus that was precipitated with 6% polyethylene glycol (Mr = 8,000)/2.2% NaCl from supernatants of virus-infected NA or BHK cell cultures. To obtain the cDNA of rabies virus glycoprotein (G) protein genes the RNA was subjected to RT-PCR. RT reactions were performed at 42°C for 1 hr with avian myeloblastosis virus reverse transcriptase (Promega) as described (14). Primer COS-5 start (5′-AAAAGACTCAAGGAAAGATG-3′) was initially used for reverse transcription of viral genomic RNA. A portion of the RT product was subjected to PCR amplification using the primers COS-5 start and COS-3 stop (5′-ATCAGGATCCTGGATCGTTGAAAGGA-3′). Amplification was carried out for 35 cycles of denaturation at 94°C for 60 sec, annealing at 50°C for 60 sec, and polymerization at 72°C for 60 sec with a Taq DNA polymerase (Promega), as described (14). The individual PCR products were purified with QIAquick PCR purification kit (Qiagen, Chatsworth, CA) and sequenced by using the AmpliTaq cycle sequencing kit (Perkin–Elmer) and several synthetic oligonucleotides as primers.
For restriction enzyme analysis, a 820-bp fragment of the G protein gene was amplified as described above by using primers C5-a (5′-CTTCACTCAAGGGTCTTC-3′) and C3-a (5′-GGAGGTGAACTTCAACAA-3′). The PCR product was digested with restriction enzyme NsiI and was subjected to electrophoresis on 1% agarose gels. The DNA was visualized by ethidium bromide staining and quantitated by densitometry with the NIH 1.58 image program. To examine for the presence of rabies virus N mRNA in muscle tissue, total RNA was isolated from the masseter muscle at different times after virus inoculation and 2.5 μg of the RNA was subjected to the RT reaction with the antisense primer RNPCR-3 (5′-CACTCAAGCCTAGTGAACGGA-3′). The PCR amplification was carried out as described above with primers RNPCR-3 and 10g-5 (5′-CTACAATGGATGCCGAC-3′). The PCR products were analyzed on 1% agarose gel, and then the gel was blotted onto a Nytran transfer membrane (Schleicher & Schuell), hybridized with 32P-labeled internal oligonucleotide probe RNseq-3 (5′-CCATAGCTGGTCCAGTCTTCC-3′), and exposed to autoradiography film. The hybridization probe was labeled with [γ-32P]ATP (ICN; specific activity, 4,500 Ci/mmol; 1 Ci = 37 GBq) by using T4 polynucleotide kinase (Promega).
Determination of Virus-Neutralizing Antibody.
The neutralization test was performed as described (12). Neutralization titers were defined as the inverse of the highest dilution of the serum under test that neutralizes 50% of the challenge virus. The mean titers were calculated for each group and the titers of each group were compared statistically by using a one-tailed t test.
RESULTS
Identification of Subpopulations of the Rabies CVS-24 Virus Strain.
As a first approach to examining the possibility that within a given rabies virus strain there are virus subpopulations that have distinguishable biological properties, we have studied whether environmental changes (i.e., growth in neuronal versus nonneuronal cells in vitro) have any effect on the nature of the virus recovered. Mouse-brain-adapted CVS-24 was passaged twice in either nonneuronal cells (BHK-21) or neuronal-type (NA) cells and the likely dominant clone from each “adapted” population was selected by limiting dilution. The clone obtained from virus passaged in NA cells was designated CVS-N2c, and the reciprocal clone from BHK cells was designated CVS-B2c. As shown in Table 1, these two clones differ significantly in their ability to infect BHK versus NA cells. Stocks were prepared from each virus clone in the corresponding cell line and then titrated in both BHK and NA cells at 34°C to obtain information concerning the relative neurotropism of the cloned viruses. As a measure of relative neurotropism, we use the following index: relative neurotropism index = logarithm of virus titer obtained in NA cells minus the logarithm of virus titer obtained in BHK cells. Although the relative neurotropism index of the CVS-N2c clone is similar to that of CVS-24, the CVS-B2c clone has a 4-fold lower relative neurotropism index, indicating a much higher capacity to grow in nonneuronal cells.
Table 1.
Relative neurotropism of variants cloned from the CVS-24 strain
| Virus | Adaptation/ selection | Titer, FFU/ml
|
Neurotropism index | |
|---|---|---|---|---|
| NA | BHK | |||
| CVS-24 | Mouse brain | 1.0 × 109 | 2.5 × 107 | 1.60 |
| CVS-N2c | NA cell | 2.8 × 105 | 4.0 × 103 | 1.85 |
| CVS-B2c | BHK cell | 1.0 × 108 | 4.5 × 107 | 0.35 |
Virus titers were determined by using the immunofluorescent antibody test described. The neurotropism index is the logarithm of the titer in NA cells minus the logarithm of the titer in BHK cells.
Because tissue tropism is predominantly a function of the glycoprotein of rabies virus, we next analyzed and compared the glycoprotein amino acid sequences deduced from the nucleotide sequences of the glycoprotein genes of the CVS-N2c and -B2c clones. Ten amino acid differences were found scattered throughout the glycoprotein sequence as shown in Fig. 1. In contrast, no difference was found between CVS-N2c and -B2c in the N protein amino acid sequence (data not shown).
Figure 1.

Amino acid sequences of the G proteins of CVS-N2c and CVS-B2c. Viral genomic RNA was isolated from virions and subjected to RT-PCR. Dots indicate conserved sequences between the two viruses.
Effect of the Host Environment on the Distribution of CVS-B2c and CVS-N2c Variants.
The differences in the nucleotide sequences of CVS-B2c and CVS-N2c have provided the means to use RT-PCR and restriction enzyme analysis to determine the relative contribution of each of these variants to the CVS-24 population as a whole. As shown in Fig. 2A, treatment of an 820-bp RT-PCR product of the glycoprotein gene of the CVS-N2c variant with the restriction enzyme NsiI results in a 410-bp fragment (Fig. 2A, lanes N2c) but the corresponding RT-PCR product of CVS-B2c is unaffected (Fig. 2A, lanes B2c). From this analysis, CVS-24 predominantly consists of virus resembling the CVS-N2c clone. CVS-24 passaged twice in NA cells (Fig. 2B, lanes N2) again resembles the CVS-N2c variant. On the other hand, CVS-24 passaged twice in BHK cells (Fig. 2B, lanes B2) consists predominantly of the CVS-B2c type. Because this pattern is highly reproducible (three parallel experiments yielded the same restriction enzyme and nucleotide sequence patterns, Fig. 2C), we conclude that the CVS-B2c variant is maintained in CVS-24 at a low level (<1%) during passage in suckling mouse brain. Under appropriate environmental pressure, such as growth in BHK cells, the CVS-B2c variant becomes dominant.
Figure 2.
Restriction enzyme analysis of the G protein genes of CVS-24, CVS-N2c, and CVS-B2c. Genomic rabies RNA was isolated from virions and a 820-bp fragment of the G protein gene was amplified by RT-PCR. (A) Digestion of the PCR product obtained from CVS-N2c with NsiI results in a 410-bp fragment, whereas the corresponding CVS-B2c PCR product is not cleaved. (B) Restriction enzyme analysis of the G protein genes of CVS-24 passaged in mouse brain (CVS-24) and after two consecutive passages in NA cells (N2) or BHK cells (B2). (C) Restriction enzyme analysis of the G protein genes of viruses obtained three parallel passaging experiments (passages 1–3).
Evidence that selection of particular variants occurs in vivo has been obtained by infection of neonatal and adult mice with mixtures of CVS-N2c and CVS-B2c at different ratios. Although replication in neonatal mice does not result in a change in the ratio of these two variants, there is strong selection for CVS-N2c in adult mice (Fig. 3). Even at a CVS-B2c/CVS-N2c input ratio of 100:1, the major population produced in the adult mouse brain is CVS-N2c. Thus selection pressures for the CVS subpopulations differ in neonatal versus adult mice. These findings substantiate the hypothesis that rabies virus strains consist of subpopulations that differ in their infectivity in vivo and in vitro.
Figure 3.
Selection for CVS-N2c of and CVS-B2c in neonatal and adult mice. Neonatal and adult mice were intracranially infected with 103 FFU of mixtures containing different ratios of CVS-N2c (N2c) and CVS-B2c (B2c). Brains were harvested from neonatal mice 4 days after infection and from adult mice 5 days after infection, total RNA was isolated from the brains, and 3 μg of total RNA was subjected to RT-PCR/restriction enzyme analysis as described in Fig. 2. After gel electrophoresis, the DNA bands were quantitated by densitometry using the nih 1.58 image program.
Pathogenesis of CVS-B2c and CVS-N2c in Adult and Neonatal Mice.
The existence of subpopulations with different biological properties within the CVS-24 strain of rabies virus has profound implications for the pathogenesis of rabies virus. To study the relationship of the CVS-24 variants with respect to the pathogenesis of rabies, we first determined the pathogenicity of CVS-B2c and CVS-N2c separately in adult and neonatal mice. As a measure of pathogenicity, we calculated a pathogenicity index as the LD50 after intracranial, intramuscular, or intradermal inoculation, divided by the virus titer (FFU) obtained in NA cells (pathogenicity index = LD50/FFUNA). In neonatal mice, the pathogenicity indices of CVS-B2c were higher than those of CVS-N2c and especially so after intramuscular infection. In adult mice, the findings were opposite; in this case the pathogenicity indices at CVS-N2c were higher, particularly after intradermal infection (Table 2). To obtain further information on the pathogenicity of the two variants, we compared the mortality kinetics of neonatal and adult mice infected with CVS-B2c or CVS-N2c virus by different routes. For each route of administration, equivalent amounts of virus were used. These data, shown in Fig. 4, demonstrate that neonatal mice succumb to infection with CVS-B2c on average 1 to 2 days earlier than infection with CVS-N2c regardless of the route of inoculation. The opposite was found to be the case for adult mice where infection with CVS-N2c was more rapidly lethal, particularly after intramuscular and intradermal infection.
Table 2.
Pathogenicity of CVS-24, CVS-N2c, and CVS-B2c
| Virus | Pathogenicity index
|
|||||
|---|---|---|---|---|---|---|
| Neonate
|
Adult
|
|||||
| I.C. | I.M. | I.D. | I.C. | I.M. | I.D. | |
| CVS-24 | ND | ND | ND | 0.11 | 0.0000021 | ND |
| CVS-N2c | 0.85 | 0.00016 | 0.00018 | 0.52 | 0.0000081 | 0.00000191 |
| CVS-B2c | 1.77 | 0.00115 | 0.00023 | 0.28 | 0.0000029 | 0.00000004 |
Pathogenicity index was calculated by dividing the LD50 value per ml determined after inoculation by the virus titer (FFU/ml). Virus titers were determined in NA cells with the immunofluorescent test and the LD50 values were calculated. I.C., Intracranial; i.m., intramuscular; i.d., intradermal; ND, not determined.
Figure 4.

Mortality of CVS-N2c and CVS-B2c in neonatal and adult mice. Neonatal mice (A–C) or adult mice (D–F) were infected intracranially with 103 FFU (A and D), intramuscularly with 105 FFU (B and E), and intradermally with 106 FFU (C and F) of CVS-N2c (▵) or CVS-B2c (○). After infection, mice were observed on a daily basis. The results are expressed as percent survivorship calculated from 10 mice per group.
The difference in susceptibility to lethal infection with CVS-B2c between adult and neonatal mice may be caused by the maturation of the immune response. In this case, CVS-B2c and CVS-N2c may differ in the ability to induce a protective immune response, possibly based on their discordant tissue tropism. To examine this possibility, mice were infected intramuscularly with 105 FFU of CVS-B2c or CVS-N2c and local replication of the viruses was assessed by RT-PCR. As can be seen in Fig. 5, N mRNA can be detected in CVS-B2c-infected muscle tissue at 24 hr after infection but N mRNA cannot be detected in CVS-N2c-infected muscle tissue until 48 hr after infection. This implies that only CVS-B2c can replicate at the site of infection immediately after inoculation. The relatively early appearance of rabies antigen in the periphery may stimulate an early immune response to the virus. As shown in Table 3, intradermal infection of adult mice with 104 FFU CVS-B2c induces a neutralizing antibody response that develops more rapidly and is significantly higher than that seen after intradermal infection with 104 FFU CVS-N2c.
Figure 5.
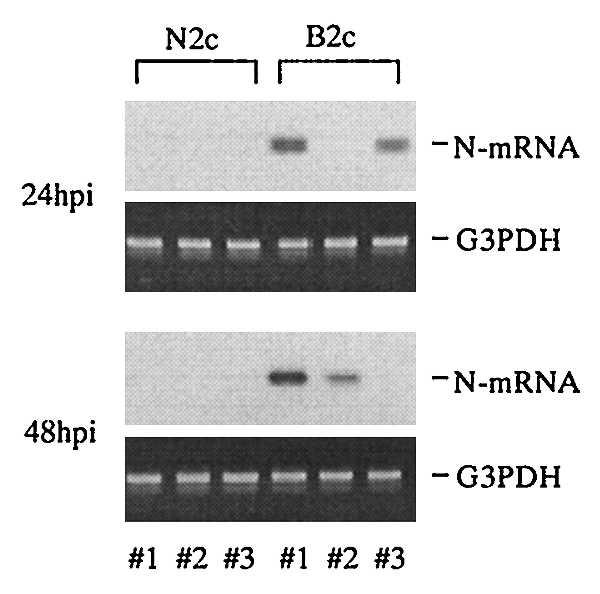
Rabies virus RNA replication in muscle tissue at the side of inoculation. Approximately 105 FFU of CVS-N2c (N2C) or CVS-B2c (B2c) was inoculated into the masseter muscle of adult mice, and at 24 and 48 hr after infection, muscle tissue was harvested from three mice (lanes 1–3), total RNA was isolated from the muscle tissue and 2.5 μg of total RNA was subjected to the RT-PCR. Rabies virus nucleoprotein mRNA was then selectively reverse-transcribed with an antisense primer, subjected to PCR amplification, and analyzed by gel electrophoresis and Southern blotting. G3PDH mRNA was RT-PCR-amplified from each RNA sample to serve as a loading control.
Table 3.
VNA response in Swiss Webster mice after intradermal infection with CVS-N2c or CVS-B2c
| Days after infection* | Titer
|
Significance | |
|---|---|---|---|
| CVS-N2c | CVS-B2c | ||
| 5 | 66.0 ± 18.3 | 102.0 ± 23.3 | 0.241 |
| 6 | 95.0 ± 20.3 | 166.0 ± 33.0 | 0.083 |
| 7 | 141.0 ± 50.6 | 414.0 ± 88.2 | 0.015 |
| 8 | 207.0 ± 46.6 | 738.0 ± 269.9 | 0.068 |
| 9 | 108.0 ± 28.0 | 837.0 ± 248.9 | 0.009 |
| 10 | 90.0 ± 24.0 | 747.0 ± 235.5 | 0.018 |
Data are the mean ± SEM of the reciprocal titers (reciprocal of the highest dilution of serum yield as a 50% reduction in test virus, as determined by the fluorescent focus inhibition test) for a group of 10 mice. The significance was determined by using the unpaired t test.
Mice were infected with 104 FFU of CVS-N2c or CVS-B2c.
DISCUSSION
Because of a high mutation rate, RNA virus strains generally consist of complex populations that can differ considerably in their genomic structure (7). Nevertheless, within a constant environment these populations remain relatively stable (7, 15). Thus the dominant rabies virus variant within a given strain, associated with a particular host, is readily identifiable and can easily be distinguished from other strains (16). Even though new variants may continuously emerge because of spontaneous mutation, a stable complex population will persevere because of fitness for a given environment. We have provided direct evidence in support of this hypothesis in our studies of the mouse-adapted rabies virus strain CVS-24. Although CVS-N2c is by far the major variant of CVS-24 when replicating in neuronal cells or central nervous system tissue, CVS-B2c rapidly becomes the dominant variant when CVS-24 is passaged in nonneuronal cells. Infection of adult mice heavily selects for CVS-N2c, whereas neonatal mice do not have preferential selective pressure for either variant.
Our results indicate that CVS-24, as it is historically maintained, is a stable but complex population. We believe that CVS-B2c is a minor, but stable, variant within the CVS-24 complex. The reproducible selection of the CVS-B2 variant, the relatively extensive amino acid differences (10 amino acids) between the glycoprotein sequence of CVS-B2c and the major CVS-24 population CVS-N2c, and the fact that CVS-11, which was adapted from mouse-brain-passaged CVS several decades ago, differs from CVS-B2c only in two amino acid residues of G sequence and phenotypic characteristics argue against the possibility that CVS-B2c evolved because of spontaneous mutation during the two in vitro passages in BHK cells that made up the selection process. These findings suggest that what are considered to be homogeneous rabies virus strains actually consist of variants with highly distinctive biological properties.
The existence of rabies virus strains as quasispecies has profound implications for the epidemiology of rabies. For example, the spillover of a rabies virus strain from one species to another may be facilitated by preexisting subpopulations that have a selective advantage in a new host. Although some strains of rabies virus are maintained in a single host species, others, such as the silver-haired bat rabies virus strain, are more promiscuous (11, 17). It may be that the latter contains variants that alone or in cooperation may have the capacity to rapidly adapt to a new host. In this case, virus isolated from an infected secondary host may differ from the virus carried by the corresponding vector species. Thus, variability in the phenotypic characteristics of street rabies viruses is conceivably the result of population dynamics within a given virus strain because of environmental changes.
The coexistence of different variants within a rabies virus strain may also contribute to the pathogenesis of rabies. Rabies virus has a rather simple genome structure without known regulatory elements but, nevertheless, must fulfill a complex infection cycle in vivo that entails the ability to replicate in neuronal and nonneuronal cells. The transition of rabies virus from the site of entry to the central nervous system and, finally, to the salivary glands most likely involves interactions with different host cell factors that may include cell surface receptors. Barriers to the spread of the virus into different tissues may be overcome by the cooperative action of virus variants with diverse tissue tropism, for example, via phenotypic mixing. In this study, we have demonstrated that a stable rabies virus strain consists of variants that differ considerably in their ability to infect neuronal and nonneuronal cells in vitro as well as in vivo. Dominance within these variants can shift rapidly in response to changing host environments. Whether or not there is any cooperation between various rabies virus variants in the natural infection remains to be proven.
ABBREVIATIONS
- CVS
challenge virus standard
- G
glycoprotein
- N
nucleoprotein
- FFU
focus-forming units
- RT-PCR
reverse transcriptase–PCR
Footnotes
References
- 1.Murphy F A, Baur S P, Harrison A K, Winn W C. Lab Invest. 1973;28:361–376. [PubMed] [Google Scholar]
- 2.Charlton K M. In: Lyssaviruses. Rupprecht C E, Dietzschold B, Koprowski H, editors. Berlin: Springer; 1994. pp. 95–119. [Google Scholar]
- 3.Ray N B, Ewalt L C, Lodmell D L. J Virol. 1995;69:764–772. doi: 10.1128/jvi.69.2.764-772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thoulouze M, Lafage M, Montano-Hirose J A, Lafon M. J Virol. 1997;71:7372–7380. doi: 10.1128/jvi.71.10.7372-7380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dockter J, Evans C F, Tishon A, Oldstone M B A. J Virol. 1996;70:1799–1803. doi: 10.1128/jvi.70.3.1799-1803.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villarete L, Somasundaram T, Ahmed R. J Virol. 1994;68:7490–7496. doi: 10.1128/jvi.68.11.7490-7496.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domingo E, Holland J J. In: RNA Genetics. Domingo E, Holland J J, Ahlquist P, editors. Boca Raton, FL: CRC; 1988. pp. 3–36. [Google Scholar]
- 8.Domingo E. Curr Opin Genet Dev. 1992;2:61–63. doi: 10.1016/s0959-437x(05)80323-5. [DOI] [PubMed] [Google Scholar]
- 9.Eigen M, Gardiner W, Schuster P, Winkler-Oswatitsch R. Sci Am. 1981;244:88–188. doi: 10.1038/scientificamerican0481-88. [DOI] [PubMed] [Google Scholar]
- 10.Novella I S, Duarte E A, Elena S F, Moya A, Domingo E, Holland J J. Proc Natl Acad Sci USA. 1995;92:5841–5844. doi: 10.1073/pnas.92.13.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rupprecht C E, Smith J S, Fekadu M, Childs J E. Emerg Infect Dis. 1995;1:107–114. doi: 10.3201/eid0104.950401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiktor T J, Macfarlan R I, Foggin C M, Koprowski H. Dev Biol Stand. 1984;57:199–211. [PubMed] [Google Scholar]
- 13.Habel K. Laboratory Techniques in Rabies. Geneva: W.H.O.; 1966. , Monograph Series no. 23, pp. 321–335. [Google Scholar]
- 14.Shankar V, Dietzschold B, Koprowski H. J Virol. 1991;65:2736–2738. doi: 10.1128/jvi.65.5.2736-2738.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quer J, Huerta R, Novella I S, Tsimring L, Domingo E, Holland J J. J Mol Biol. 1996;264:465–471. doi: 10.1006/jmbi.1996.0654. [DOI] [PubMed] [Google Scholar]
- 16.Smith J S. Clin Microbiol Rev. 1996;9:166–176. doi: 10.1128/cmr.9.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morimoto K, Patel M, Corisdeo S, Hooper D C, Fu Z F, Rupprecht C E, Koprowski H, Dietzschold B. Proc Natl Acad Sci USA. 1996;93:5653–5658. doi: 10.1073/pnas.93.11.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]


