Abstract
Background
Action potential generation in excitable cells such as myocytes and neurons critically depends on voltage-gated sodium channels. In mammals, sodium channels exist as macromolecular complexes that include a pore-forming alpha subunit and 1 or more modulatory beta subunits. Although alpha subunit genes have been cloned from diverse metazoans including flies, jellyfish, and humans, beta subunits have not previously been identified in any non-mammalian species. To gain further insight into the evolution of electrical signaling in vertebrates, we investigated beta subunit genes in the teleost Danio rerio (zebrafish).
Results
We identified and cloned single zebrafish gene homologs for beta1-beta3 (zbeta1-zbeta3) and duplicate genes for beta4 (zbeta4.1, zbeta4.2). Sodium channel beta subunit loci are similarly organized in fish and mammalian genomes. Unlike their mammalian counterparts, zbeta1 and zbeta2 subunit genes display extensive alternative splicing. Zebrafish beta subunit genes and their splice variants are differentially-expressed in excitable tissues, indicating tissue-specific regulation of zbeta1-4 expression and splicing. Co-expression of the genes encoding zbeta1 and the zebrafish sodium channel alpha subunit Nav1.5 in Chinese Hamster Ovary cells increased sodium current and altered channel gating, demonstrating functional interactions between zebrafish alpha and beta subunits. Analysis of the synteny and phylogeny of mammalian, teleost, amphibian, and avian beta subunit and related genes indicated that all extant vertebrate beta subunits are orthologous, that beta2/beta4 and beta1/beta3 share common ancestry, and that beta subunits are closely related to other proteins sharing the V-type immunoglobulin domain structure. Vertebrate sodium channel beta subunit genes were not identified in the genomes of invertebrate chordates and are unrelated to known subunits of the para sodium channel in Drosophila.
Conclusion
The identification of conserved orthologs to all 4 voltage-gated sodium channel beta subunit genes in zebrafish and the lack of evidence for beta subunit genes in invertebrate chordates together indicate that this gene family emerged early in vertebrate evolution, prior to the divergence of teleosts and tetrapods. The evolutionary history of sodium channel beta subunits suggests that these genes may have played a key role in the diversification and specialization of electrical signaling in early vertebrates.
Background
Coordinated electrical signals in the metazoan nervous system, heart, and skeletal muscle depend on the generation of action potentials, rapid changes in membrane potential mediated by the passage of ions through voltage-gated ion channels [1]. The initial upstroke of the action potential in most excitable cells is determined by sodium channels, membrane proteins characterized by rapid activation and inactivation and high ionic conductance [1-3]. Unlike evolutionarily-ancient, tetrameric potassium channels, sodium channels are comprised of four homologous domains that are encoded by a single polypeptide [1-3]. Sodium channels are suspected to have evolved from structurally-similar calcium channels, which in turn likely arose following the duplication of potassium channel genes [1-3]. The use of sodium as a conducting ion rather than calcium is thought to have permitted rapid conduction and high-frequency electrical signaling in the rudimentary nervous systems of early multicellular animals, without the numerous additional intracellular effects mediated by calcium's role as a second messenger [1,4].
In mammals, voltage-gated sodium channels are multi-protein complexes that include a pore-forming α subunit and 1 or more modulatory β subunits [5-9]. While sodium channel α subunits are large proteins with 24 membrane-spanning domains, β subunits are smaller proteins with a single transmembrane domain and an extracellular V-type immunoglobulin (IG)-like motif [10-14]. Ten distinct α subunit genes (SCNxA) and 4 β subunit genes (SCN1B-4B) have been cloned from mammals, and all have been functionally expressed with the exception of SCN7A [2,3,15]. While heterologous expression of α subunit genes alone can reconstitute key properties of voltage-gated sodium channels observed in native tissues, co-expression with β subunit genes modifies channel gating and increases channel cell surface expression [10-13]. Studies of mice with targeted ablation of either β1 or β2 genes corroborated these roles for β subunits in vivo and revealed an additional function for β subunits in α subunit localization [16,17]. Moreover, the unique expression profiles of each β subunit gene and their variable effects on α subunit function, expression, and localization suggest that different subunit combinations in heart, brain, and muscle may contribute to diversity and specialization in electrical signaling [18,19].
The physiological importance of sodium channel α and β subunits is reinforced by their role in human disease. Mutations that cause even subtle changes in sodium channel α subunit function may result in severe human disease phenotypes including epilepsy and arrhythmia [20]. To date, mutations in 7 α subunit genes (SCN1A, SCN2A, SCN3A, SCN4A, SCN5A, SCN8A, SCN9A) have been linked to human disease [21-27]. Given their important modulatory effects on the expression and function of sodium channel α subunits, β subunits are also important candidate genes for human diseases related to sodium channel dysfunction. β1 subunit gene mutations are a cause of generalized epilepsy with febrile seizures plus (GEFS+), and variants in sodium channel α subunits that disrupt their interaction with β subunits may be pathological in the heart [28-31]. More recently, a mutation in the β4 subunit gene was implicated as a cause of the Long QT syndrome, a congenital arrhythmia [32].
The relatively large number of distinct mammalian sodium channel α subunit isoforms has generated great interest in the evolution of this gene family, particularly since invertebrate genomes contain far fewer conserved Nav1 sodium channel genes [33-36]. The physical linkage of voltage-gated sodium channel genes to the 4 homeobox (HOX) gene clusters suggests that polyploidization at least partly contributed to the emergence of multiple ancestral sodium channel genes in early vertebrates [37-39]. Moreover, the clustering of closely-related sodium channel genes in mammalian genomes (e.g. SCN1A, SCN2A, and SCN3A on human chromosome 2) is consistent with the hypothesis that several of the 10 mammalian isoforms arose following tandem duplication of ancestral vertebrate genes [36-39]. Since fish and mammals possess different numbers of sodium channel isoforms, the duplication of ancestral vertebrate sodium channel genes is suspected to have occurred independently in teleost and tetrapod lineages [38]. Expansion of the sodium channel gene family by tandem duplication may also have occurred uniquely in tetrapods, since the complement of sodium channel genes in teleosts appears to have resulted from whole-genome duplication [40,41].
The unique electrophysiological properties, expression patterns, and physiological roles of mammalian sodium channel α subunits suggest that the duplication of these genes may have been an adaptation that permitted the diversification and functional specialization of electrical signaling in vertebrates [1,39,42]. The physical interaction of α subunits with auxiliary proteins such as β subunits that modify their expression or function may have been similarly adaptive [36,37]. Although sodium channel β subunits have not previously been identified in non-mammalian species, we hypothesize that a sodium channel macromolecular complex comprised of α subunits and auxiliary β subunits is an evolutionarily-conserved structural entity. Evidence for the emergence of sodium channel β subunit genes early in vertebrate evolution would be consistent with the idea that these genes may have played a role in diversifying and fine-tuning electrical signaling not only in mammals but in all vertebrates.
Here we report the identification and molecular cloning of conserved orthologs of all 4 mammalian β subunit genes in Danio rerio (zebrafish), a teleost vertebrate and a popular developmental and physiological model system. By direct sequence analysis and alignment, we assessed whether zebrafish β subunits possess the structural hallmarks of their mammalian counterparts. We provide evidence for extensive splicing of zebrafish beta subunit genes and demonstrate their expression in excitable tissues. To determine whether zebrafish sodium channel α and β subunits functionally interact, we studied sodium currents generated by the co-expression of the genes encoding zNav1.5 (α subunit) and zβ1 in a heterologous cell system. Finally, we assessed the synteny and phylogeny of β subunits and a group of closely-related genes with IG domains in several vertebrate species. Our findings strongly suggest that all 4 voltage-gated sodium channel β subunit genes emerged early in vertebrate evolution, and support the concept that the voltage-gated sodium channel α-β subunit macromolecular complex is a conserved and functionally-important vertebrate innovation.
Results
Identification and cloning of zebrafish sodium channel β subunit genes
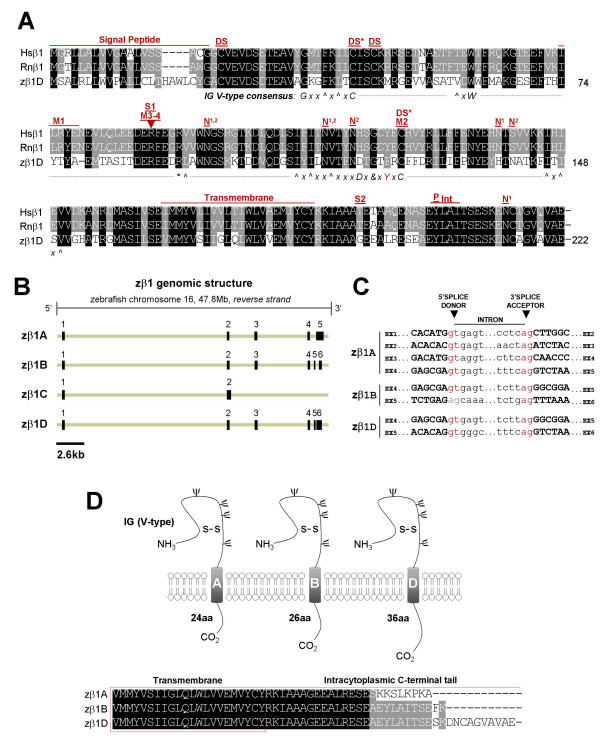
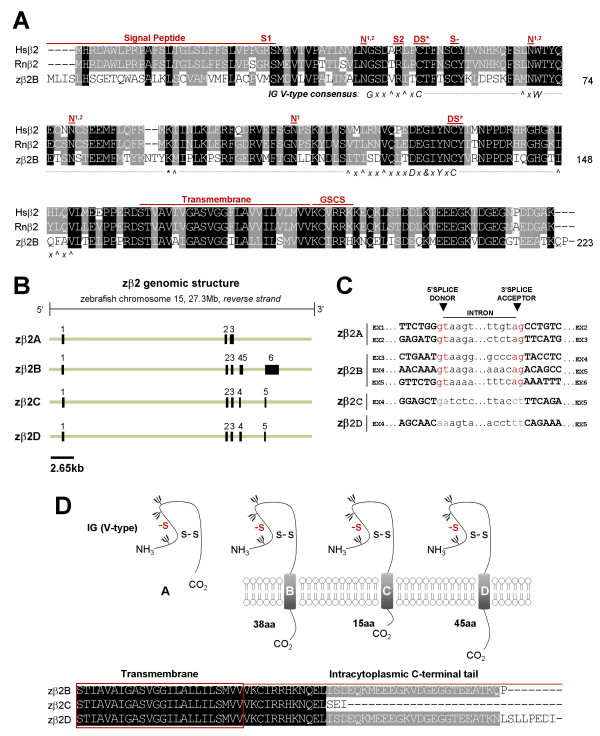
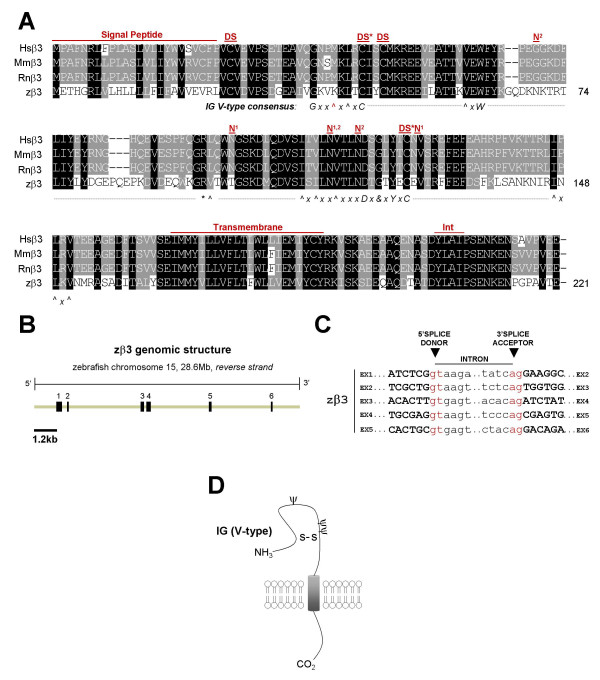
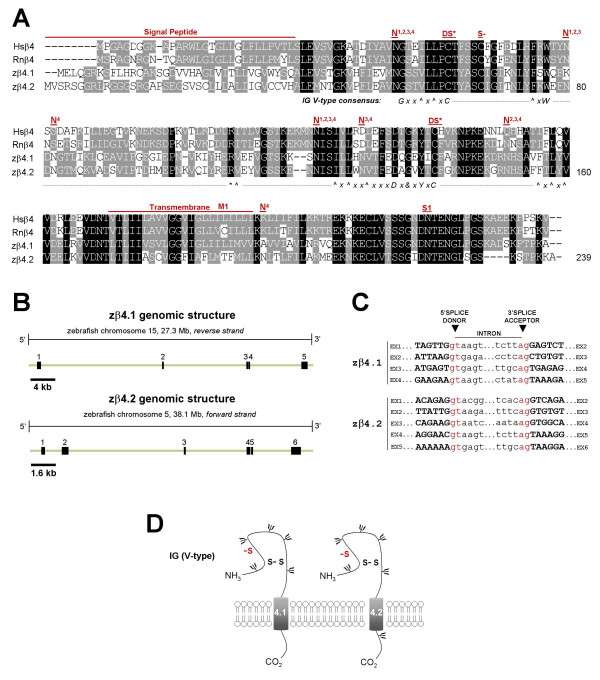
We identified 5 distinct zebrafish β subunit gene loci by mining the draft zebrafish genome sequence (ensembl Zv2-4) for genes homologous to the human and mouse β1- β4 genes. In silico prediction of coding sequence on the relevant genomic DNA contigs enabled us to amplify partial β subunit gene sequences for each locus by RT-PCR and subsequently complete the full-length zebrafish β subunit clones by rapid amplification of cDNA ends (RACE)-PCR. Analysis of putative open reading frames within each cDNA sequence predicted proteins ranging from 220–232 amino acids in length. Deduced translation and alignment of the five zebrafish amino acid sequences with each mammalian β subunit protein sequence suggested the identities of these genes (Figs. 1A, 2A, 3A, 4A). While single homologs were identified for the genes encoding β1-β3 (zβ1-zβ3), zebrafish express two distinct but closely-related β4 genes (zβ4.1, zβ4.2). Alignment of the amino acid sequences of zebrafish β1, β2, β3, β4.1, and β4.2 with their human homologs indicated that these proteins share 53.4%, 49.6%, 51.1%, 43.2%, and 40.8% identity, respectively.
Figure 1.
Analysis of the cloned zebrafish β1 subunit gene and novel splice variants. A) Alignment of cloned human, rat, and zebrafish β1 amino acid sequences. Black = identical in all three species; grey = identical in 2/3 species or conserved substitution. Shown for zebrafish is the most conserved β1 splice form (variant D). Hs = Homo sapiens, Rn = Rattus norvegicus, z = zebrafish; DS* = cysteine residue predicted to participate in a disulfide bridge, based on the myelin P0 protein crystal structure; DS = predicted second disulfide bridge; N = predicted N-linked glycosylation site (N1 = human/rat, N2 = zebrafish); M1 = site of epilepsy-causing deletion (I70_E74del) in Hsβ1; M2 = site of second epilepsy-causing mutation (C121W) in Hsβ1; M3/4 = site of third and fourth epilepsy-causing mutations (R85C, R85H); S1 = nonsynonymous Hsβ1 single nucleotide polymorphism (SNP, G/A > R85H); S2 = nonsynonymous Hsβ1 SNP (C/T > T189M); P = phosphorylation site (tyrosine Y181) that regulates ankyrin recruitment (NOTE: Y200 = Y181 following cleavage of 19 amino acid signal peptide); IN = putative internalization sequence. Consensus sequence for V-type IG domain is depicted beneath the alignment: G = glycine, x = any residue, ^ = hydrophobic residues, C = cysteine, - = gap in alignment with consensus sequence, W = tryptophan, * = basic residue, L = leucine, D = aspartic acid, & = glycine, alanine, or aspartate, and Y = tyrosine. Red indicates zebrafish residues that deviate from the consensus sequence. See Results for references supporting sequence annotation. B) 5' and 3' RLM-RACE PCR and RT-PCR identified four distinct splice variants expressed from zβ1 locus on zebrafish chromosome 16 (Ensembl). C) Splice donor and acceptor sites of zebrafish β1 splice variants, derived from comparing cloned cDNA against genomic DNA sequences (Ensembl). Consensus GT-AG splice sites are labeled in red. A splice-site deviating from the consensus appears in grey. D) Schematic diagram of β1 splice variants A, B, and D, whose predicted proteins differ only in the length of their intracytoplasmic C-terminal tail. S-S = disulfide bridge. NH3 = 5' amino terminus, CO2 = 3' carboxyl terminus, β = putative N-linked glyosylation site. Alignment of C-terminal tail of variants zβ1A, zβ1B, and zβ1D (below). zβ1C is not shown as it is predicted to lack both extracellular IG and transmembrane domains.
Figure 2.
Analysis of the cloned zebrafish β2 subunit gene and novel splice variants. Presentation and labeling as in Figure 1. A) Alignment of cloned human, rat, and zebrafish β2 amino acid sequences. Shown for zebrafish is the most conserved β2 splice form (variant B). S- = conserved cysteine in β2 that is a putative site of covalent linkage with a partner α subunit; S1 = nonsynonymous Hsβ2 single nucleotide polymorphism (SNP, C/T > R28W) (NCBI dbSNP, PharmGKB); S2 = nonsynonymous Hsβ2 SNP (G/A > R47H) (NCBI dbSNP, PharmGKB); GSCS = γ-secretase cleavage site. B) 5' and 3' RLM-RACE PCR and RT-PCR identified four distinct splice variants expressed from the zβ2 locus on zebrafish chromosome 15 (Ensembl). C) Splice donor and acceptor sites of zebrafish β2 splice variants. Zβ2 variants C and D both differ from the consensus sequences at the exon 4-intron 4 and intron 4-exon 5 splice junctions. D) Schematic diagram of β2 splice variants A-D. With the exception of variant A, the predicted proteins of zβ2 variants B-D differ only in the length of their intracytoplasmic C-terminal tail. Alignment of C-terminal tail of variants zβ2B, zβ2C, and zβ2D (below).
Figure 3.
Analysis of the cloned zebrafish β3 subunit gene. Presentation and labeling as in Figure 1. A) Alignment of cloned human, mouse, rat, and zebrafish β3 amino acid sequences. INT = putative internalization sequence. B) 5' and 3' RLM-RACE PCR and RT-PCR identified a single transcript expressed from the zβ3 locus on zebrafish chromosome 15 (Ensembl). zβ3 has six exons. C) All zβ3 splice sites adhere to GT-AG consensus sequences. D) Schematic diagram of the β3 protein.
Figure 4.
Analysis of cloned zebrafish β4.1 and β4.2 subunit genes. Presentation and labeling as in Figure 1. A) Alignment of cloned human, rat, and zebrafish β4 amino acid sequences. S- = conserved cysteine in β4 that is a putative site of covalent linkage with a partner α subunit; N = predicted N-linked glycosylation site (N1 = human, N2 = rat, N3 = zebrafish β4.1, N4 = zebrafish β4.2); M = site of putative Long QT syndrome-causing mutation L179F; S1 = nonsynonymous Hsβ4 SNP (A/C > N210H). B) Genomic organization of zβ4.1 and zβ4.2 derived from comparing cloned cDNA sequences with genomic sequences of zebrafish chromosomes 15 and 5, respectively. zβ4.1 has five exons and zβ4.2 has six exons. C) All zβ4.1 and zβ4.2 splice sites exhibit consensus GT-AG donor/acceptor sequences. D) Schematic diagram of zβ4.1 and zβ4.2 proteins.
Conserved features of zebrafish sodium channel β subunits
Mammalian sodium channel β subunits possess a single transmembrane domain, a short intracellular carboxyl C-terminal tail, a cleavable amine (N)-terminal signal peptide, and a conserved V-type extracellular IG motif that most closely resembles the type found in cell-adhesion molecules [14,43]. Hydropathy analysis of zebrafish β subunit amino acid sequences using TMpred software revealed that all 5 proteins are likely to possess cleavable N-terminal signal peptides and single transmembrane domains (additional file 1). To assess the presence of an extracellular V-type IG domain, we first analyzed each zebrafish beta subunit amino acid sequence with the NCBI conserved domain database. By this method, all 5 zebrafish β subunit genes are predicted to possess V-type extracellular IG domains (additional file 2). To more rigorously analyze these predictions, we manually examined the amino acid sequence of each zebrafish β subunit for the V-type IG domain consensus sequence (annotated beneath alignments, Figs. 1A, 2A, 3A, 4A). With the exception of a conservative tyrosine to phenylalanine (Y > F) substitution in zβ1 and a non-polar methionine to basic lysine residue substitution (M > K) in zβ3 (illustrated in red text in Figs. 1A and 3A), all 5 zebrafish β subunits exhibit 100% conservation of the consensus sequence for V-type IG domains [14,43]. Typical of this domain is the linkage of 2 cysteine residues in a disulfide bridge (DS*, Figs. 1A, 2A, 3A, 4A), the structural importance of which was revealed by a mutation that results in familial epilepsy [28]. A second putative disulfide bridge that may further stabilize the extracellular IG domain in β1 and β3 is suggested of the crystal structure of myelin protein zero (MPZ), the primary structural protein of peripheral nerve myelin, whose IG domain closely resembles that found in sodium channel β subunits [12,43,44]. The cysteine residues that contribute to proposed second bridge are conserved in zβ1 and zβ3 (DS, Figs. 1A, 3A). Similar to mammalian β2 and β4, zβ2 and zβ4 proteins do not possess this second disulfide bridge but have an unpaired cysteine residue that may underlie covalent interactions with partnering sodium channel α subunits (S-, Figs. 2A, 4A) [5,11,13].
In addition to V-type IG domains, our sequence analysis indicated that other functional and/or structural domains are conserved in zebrafish β subunits (Figs. 1A, 2A, 3A, 4A). These include a tyrosine in β1 that is phosphorylated and regulates interactions with ankyrin G (Y181 or Y200 with signal peptide intact) [45], the C-terminal tyrosine-leucine-alanine-isoleucine (Y-L-A-I) internalization motif in β1 and β3 which may be recognized by clathrin-coated pits [12], and a juxtatransmembrane gamma-secretase cleavage site in β2 that may play a role in cell adhesion or the movement of cells expressing this subunit [46]. Zebrafish β subunits are also likely to exhibit post-translational modification by amide nitrogen (N)-linked glycosylation, a key feature of all 4 mammalian β subunits [10-13]. The zβ1 protein possesses 2 of 4 predicted N-linked glycosylation sites found in human or rat β1 in addition to exhibiting two unique sites (Fig. 1A). Similarly, zβ2 conserves 3 of 4 sites, zβ3 conserves 1 of 3 sites and displays 2 novel sites, zβ4.1 conserves 4 sites and displays 1 novel site, and zβ4.2 conserves 3 of four sites and displays 3 novel sites (Figs. 2A, 3A, 4A).
Comparative genomics of β subunit gene variants
The conservation of amino acid residues between homologous genes in distantly-related species may uncover previously unappreciated regions of functional importance and facilitate the evaluation of novel human mutations and polymorphisms. To determine whether previously identified mutations and polymorphisms are conserved in zebrafish β subunit genes, we mapped these variants onto the alignments of mammalian and zebrafish β subunit amino acid sequences. Four mutations in the human β1 gene have been linked to the heritable epilepsy syndrome GEFS+, the first resulting in a 5 amino acid deletion from isoleucine at position 70 to glutamic acid at position 74 (I70_E74del), the second in substitution of a cysteine residue that participates in a disulfide bridge (C121W), and the third and fourth resulting in substitution of an arginine at position 85 for either a cysteine or a histidine, respectively (R85C, R85H) [28,47,48]. All of these mutations are expected to destabilize the extracellular IG domain of β1 and result in loss of function. In zβ1, 2 of 5 amino acids in the deleted segment (isoleucine, tyrosine), C121, and R85 are all conserved (M1, M2, M3/4, Fig. 1A). R85H was also previously reported as a non-synonymous SNP in NCBI and PharmGKB SNP databases (S1, Fig. 1A). A second reported SNP in human β1 results in a threonine to methionine substitution at position 189 (T189M), but T189 is not conserved in the amino acid sequence of zβ1 (S2, Fig. 1A).
Although disease-causing mutations are not currently associated with either SCN2B or SCN3B, nonsynonymous SNPs have been reported for SCN2B that result in an arginine to tryptophan substitution at position 28 (R28W) and an arginine to histidine substitution at position 47 (R47H). R47 but not R28 is conserved in the amino acid sequence of zebrafish β2 (S1, S2, Fig. 2A). A mutation in SCN4B resulting in a leucine to phenylalanine substitution at position 179 (L179F) was recently implicated as a putative cause of the congenital Long QT syndrome [32]. Although this leucine is conserved in zebrafish β4.1, rat β4 and zebrafish β4.2 display a cysteine (C) and threonine (T) at this position, respectively (M1, Fig. 4A). A nonsynonymous SCN4B polymorphism has also been reported, resulting in an asparagine to histidine substitution at position 210 (N210H). N210 is conserved in the sequences of both zβ4.1 and zβ4.2 (S1, Fig 4A).
Alternative splicing of zebrafish β subunit genes
The mammalian β1 subunit gene is alternatively-spliced in both rats (β1A) and humans (β1B), with retention of intron 3 being the primary event in both species that dramatically alters the transmembrane domain and intracellular C-terminus of the β1 subunit protein [49,50]. An alternative splice-variant of β1 that retains intron 5 and adds 86 nucleotides to the 3' untranslated region (UTR) of β1 mRNA transcripts has also been reported [51,52]. In zebrafish, we detected 4 alternatively-spliced variants of the β1 gene, designated here as A-D. Comparisons between complementary DNA and genomic DNA revealed that zβ1A transcripts have 5 exons while zβ1B and zβ1D each have 6 exons and zβ1C has only 2 (Fig. 1B, Table 1). Transcripts of zβ1A, zβ1B, and zβ1D all share exons 1 through 4 but not exons 5 and 6; and all splice junctions except exon 5-intron 5 of zβ1B display canonical GT-AG splice donor-acceptor sites (Figs. 1B, 1C; Table 1). The zβ1 splice variant that shares the most identity with human β1 at the amino acid level (zβ1D) also shares nearly identical genomic organization (Table 1). With the exception of differences in exons 5 and 6, zβ1A and zβ1B variants also share this conserved genomic architecture (Table 1).
Table 1.
Comparative genomics of the sodium channel β1 gene in zebrafish and mammals.
| gene | Genbank accession number | gene location | cDNA | exon1 | exon2 | exon 3 | exon 4 | exon 5 | exon6 | protein | %ID (%SIM) | topology |
| zβ1A | DQ489722 | Chr 16 | 1411 | 219 (52) | 167 | 238 | 142 | 645 (31) | - | 209 | 47.5% (57.0%) | IG(V): 37–149 TM: 164–185 |
| zβ1B | DQ489723 | Chr 16 | 1054 | 219 (52) | 167 | 238 | 142 | 28 | 260 (9) | 211 | 49.8% (59.6%) | IG(V): 37–149 TM: 164–185 |
| zβ1C | DQ489724 | Chr 16 | 574 | 219 (52) | 355 (266) | - | - | - | - | 105 | 13.0% (17.5%) | IG(V): none TM: none |
| zβ1D | DQ489725 | Chr 16 | 1320 | 219 (52) | 167 | 238 | 142 | 72 (67) | 482 (0) | 221 | 53.4% (63.7%) | IG(V): 37–149 TM: 164–185 |
| hβ1 | NM_001037 | Chr 19 | 1521 | 231 (40) | 167 | 241 | 142 | 72 (67) | 668 (0) | 218 | 100% (100%) | IG(V): 33–146 TM: 161–182 |
| rβ1A | AF182949 | Chr 1 | 850 | 40 | 167 | 643 (615) | - | - | - | 273 | 55.1% (59.1%) | IG(V): 33–146 TM: 216–234 |
| hβ1B | NM_199037 | Chr 19 | 1170 | 231 (40) | 167 | 772 (600) | - | - | - | 268 | 58.0% (60.2%) | IG(V): 33–146 TM: 244–263 |
Gene: z = zebrafish, h = human, r = rat, and letters A-D refer to different splice variants. Accession numbers are identifiers for nucleotide sequences in NCBI GenBank. Values for cDNA and exons represent number of nucleotides. (Parentheses) indicate nucleotides within the open reading frame (difference = untranslated sequence or UTR). Protein values represent amino acid number with signal peptide intact. %ID (%SIM) refers to percentage identity and similarity with the human β1 protein sequence as determined by alignment. Zebrafish β subunit topology was determined as described in Methods. IG(V) = V-type immunoglobulin domain and TM = transmembrane domain. Numbering refers to β1 protein sequence with signal peptide intact. IG(V) domain and transmembrane regions for human β1 and human/rat splice variants were annotated based on previous reports [10, 14, 43, 49, 50].
Similar to splice variants of the mammalian β1 gene, splice variants of zβ1 are all predicted to encode proteins with variable C-termini. Unlike the mammalian variants, however, zβ1A, zβ1B, and zβ1D are predicted to possess identical transmembrane domains and differ only in their distal C-termini (Fig. 1D). As a result of alternative-splicing, zβ1A lacks the conserved tyrosine residue (Y200, Fig. 1A) that was found to regulate recruitment of ankyrin G by mammalian β1, as well as the putative internalization sequence (Y-L-A-I) discussed above (Figs. 1A, 1D) [12,45,53]. These alterations may affect interactions between zβ1A and zebrafish ankyrin, as well as influence the cycling of the mature zβ1A protein from its membrane compartment. The zβ1C splice variant, which results from retention of intron 2 and has an open reading frame of only 105 amino acids, is predicted to lack both an extracellular IG motif and a membrane-spanning segment.
Although alternative splicing of the β2 gene has not previously been identified in mammals, we identified 4 unique β2 transcripts in zebrafish. While zβ2A and zβ2B are spliced at canonical splice donor and acceptor sites, variants zβ2C and zβ2D are assembled by splicing at non-canonical exon 4-intron 4 and intron 4-exon 5 splice donor and acceptor sites. zβ2A is assembled from only 3 exons, zβ2B from 6 exons, and zβ2C and zβ2D from 5 exons each (Fig. 2B). Mammalian and zebrafish β2 genes all share a second exon that is 167 nucleotides in length and encodes the initial segment of the subunit's extracellular IG domain. Otherwise, the genomic organization of zβ2 splice variants diverges from that of human, mouse, and rat β2 genes which all have 4 coding exons (Table 2). Nevertheless, exons 1 and 3 are similar in length in all species, as is the coding segment of exon 4 in the mammalian β2 genes and the zβ2B and zβ2D splice variants (Table 2). zβ2B, zβ2C, and zβ2D are unique in possessing a fifth exon that contributes to the gene's open reading frame. Similar to mouse β2, the most conserved zebrafish β2 splice variant at the amino acid level (zβ2B) has a lengthy terminal exon that is entirely non-coding.
Table 2.
Comparative genomics of the sodium channel β2 gene in zebrafish and mammals.
| gene | Genbank accession number | gene location | cDNA | exon 1 | exon 2 | exon 3 | exon 4 | exon 5 | exon 6 | protein | %ID (%SIM) | topology |
| zβ2A | DQ489726 | Chr 15 | 821 | 243 (82) | 167 | 411 (234) | - | - | - | 160 | 32.6% (42.5%) | IG(V): 47–152 TM: none |
| zβ2B | DQ489727 | Chr 15 | 2449 | 243 (82) | 167 | 217 | 196 | 68 (10) | 1558 (0) | 223 | 49.6% (64.7%) | IG(V): 47–152 TM: 162–186 |
| zβ2C | DQ489728 | Chr 15 | 851 | 243 (82) | 167 | 217 | 124 | 100 (13) | - | 201 | 45.2% (58.4%) | IG(V): 47–152 TM: 162–186 |
| zβ2D | DQ489729 | Chr 15 | 918 | 243 (82) | 167 | 217 | 193 | 98 (34) | - | 231 | 48.1% (62.8%) | IG(V): 47–152 TM: 162–186 |
| Hsβ2 | NM_004588 | Chr 11 | 4939 | 260 (70) | 167 | 211 | 4301 (200) | - | - | 215 | 100% (100%) | IG(V): 43–146 TM: 156–180 |
| Mmβ2 | NM_001014761 | Chr 9 | 3980 | 213 (70) | 167 | 211 | 618 (200) | 2771 (0) | - | 215 | 92.1% (94.4%) | IG(V): 43–146 TM: 156–180 |
| Rnβ2 | NM_012877 | Chr 8 | 873 | 236 (70) | 167 | 211 | 259 (200) | - | - | 215 | 93.0% (94.9%) | IG(V): 43–146 TM: 156–180 |
Presentation and labeling as in Table 1. %ID (%SIM) refers to percentage identity and similarity with the human β2 protein sequence as determined by alignment. Zebrafish β2 subunit topology was determined as described in Methods. Numbering refers to β2 protein sequence with signal peptide intact. IG(V) domain and transmembrane regions for human β2 and human/mouse/rat splice variants were annotated based on previous reports [11, 14, 43, 101].
As observed for zβ1, the conceptual translation of zβ2 splice variants predict zβ2 proteins that are of variable length and amino acid sequence at their intracellular C-terminal tail (Fig. 2D). The variant zβ2A results from retention of intron 3 and is predicted to result in a 160 amino acid protein that has an intact extracellular IG domain but no transmembrane domain (Fig. 2D). zβ2A is thus unlikely to display canonical beta subunit activities such as modulation of the trafficking and/or function of voltage-gated sodium channels α subunits, or mediation of cell-adhesion between cells expressing this protein and other cells or the extracellular matrix, which all depend on integration in the cell membrane. Transcripts for variants zβ2B-D result from alternative splicing of exons 4–6 and are expected to produce proteins of 223, 201, and 231 amino acids, respectively (Fig. 2D, Table 2). Since the function of the intracellular C-terminal tail of zβ2 is not well-characterized, the predicted impact of these splice variants on zβ2 function cannot be readily predicted.
Unlike the zβ1 and zβ2 genes, alternatively-spliced transcripts of zβ3, zβ4.1, and zβ4.2 genes were not detected. For each of these three genes, the genomic organization is well-conserved compared to that of its respective mammalian homolog (Tables 3, 4). The open reading frame of SCN3B in humans, mice, rats, and zebrafish is derived from 5 exons (exons 2–6), with a non-coding initial exon found in all 4 species and a non-coding terminal exon found only in the mammalian β3 gene (Table 3). Splicing of zebrafish β3 is determined by canonical GT-AG splice donor and acceptor sites, producing a putative protein whose secondary structure is similar to the β3 subunit found in mammals (Figs. 3C, D). zβ4.1 and zβ4.2 transcripts are comprised of 5 and 6 exons, respectively, all of which are assembled from splicing at canonical GT-AG splice donor and acceptor sites (Fig. 4B, C). zβ4.2 is unique among β4 genes in possessing a non-coding exon 1 (Table 4). The predicted secondary structures of zβ4.1 and zβ4.2 are both similar to the β4 subunit found in mammals (Fig. 4D).
Table 3.
Comparative genomics of the sodium channel β3 gene in zebrafish and mammals.
| gene | Genbank accession number | gene location | cDNA | exon 1 | exon 2 | exon 3 | exon 4 | exon 5 | exon 6 | exon 7 | protein | %ID (%SIM) | topology |
| zβ3 | DQ489730 | Chr 15 | 1006 | 294 (0) | 103 (55) | 170 | 235 | 139 | 65 (64) | - | 220 | 51.1% (65.2%) | IG(V): 38–150 TM: 165–186 |
| Hsβ3 | NM_018400 | Chr 11 | 4052 | 778 (0) | 80 (55) | 164 | 226 | 139 | 86 (64) | 2579 (0) | 215 | 100% (100%) | IG(V): 38–145 TM: 160–181 |
| Mmβ3 | NM_178227 | Chr 9 | 3549 | 206 (0) | 80 (55) | 164 | 226 | 139 | 80 (64) | 2654 (0) | 215 | 97.7% (97.7%) | IG(V): 38–145 TM: 160–181 |
| Rnβ3 | NM_139097 | Chr 8 | 3910 | 350 (0) | 82 (55) | 164 | 226 | 139 | 80 (64) | 2837 (0) | 215 | 98.1% (98.1%) | IG(V): 38–145 TM: 160–181 |
Presentation and labeling as in Table 1. %ID (%SIM) refers to percentage identity and similarity with the human β3 protein sequence as determined by alignment. Zebrafish β3 subunit topology was determined as described in Methods. Numbering refers to β3 protein sequence with signal peptide intact. IG(V) domain and transmembrane regions for human β3 and human/mouse/rat splice variants were annotated based on previous reports [12, 14].
Table 4.
Comparative genomics of the sodium channel β4 gene in zebrafish and mammals.
| gene | Genbank accession number | gene location | cDNA | exon -1 | exon 1 | exon 2 | exon 3 | exon 4 | exon 5 | protein | %ID (%SIM) | topology |
| zβ4.1 | DQ489731 | Chr 22 | 2072 | - | 597 (91) | 164 | 220 | 130 | 961 (94) | 232 | 43.2% (59.3%) | IG(V): 53–155 TM: 165–187 |
| zβ4.2 | DQ489732 | Chr 5 | 1490 | 233 (0) | 410 (97) | 164 | 223 | 130 | 330 (85) | 232 | 40.8% (56.7%) | IG(V): 55–158 TM: 168–190 |
| Hsβ4 | NM_174934 | Chr 11 | 4489 | - | 214 (61) | 173 | 229 | 130 | 3743 (94) | 228 | 100% (100%) | IG(V): 46–151 TM: 162–183 |
| Mmβ4 | NM_001013390 | Chr 9 | 4244 | - | 61 | 173 | 229 | 130 | 3651 (94) | 228 | 79.4% (88.2%) | IG(V): 46–151 TM: 162–183 |
| Rnβ4 | NM_001008880 | Chr 8 | 4272 | - | 61 | 173 | 229 | 130 | 3679 (94) | 228 | 80.3% (88.6%) | IG(V): 46–151 TM: 162–183 |
Presentation and labeling as in Table 1. %ID (%SIM) refers to percentage identity and similarity with the human β4 protein sequence as determined by alignment. Zebrafish β4 subunit topology was determined as described in Methods. Numbering refers to β4 protein sequence with signal peptide intact. IG(V) domain and transmembrane regions for human β4 and human/mouse/rat splice variants were annotated based on previous reports [13, 14].
Tissue-specific regulation of zebrafish β subunit gene expression and splicing
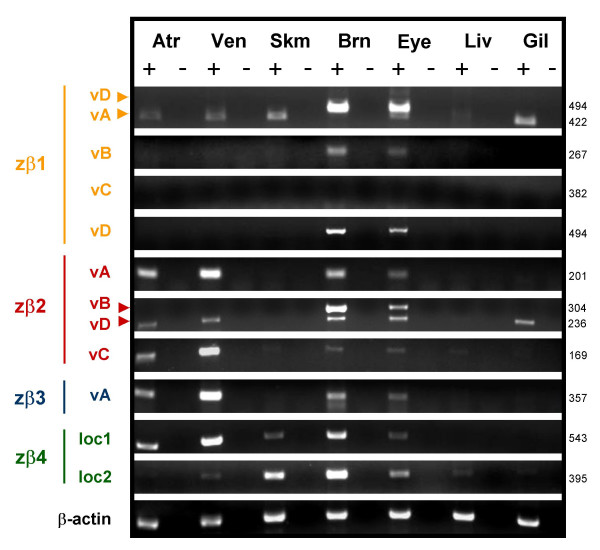
Mammalian sodium channel β subunit genes are expressed primarily in excitable tissues such as the heart, brain, and skeletal muscle. By RT-PCR, we observed that zebrafish β subunit genes are also expressed in excitable tissues where they may act to regulate the expression or function of sodium channel α subunits (Fig. 5, Table 5). Moreover, we detected distinct expression patterns for zβ1-4, suggesting that the functional importance of each β subunit likely varies by tissue type. Transcripts of zβ2 or zβ3 are absent from skeletal muscle while zβ1 and zβ4.2 are expressed only at low levels in the heart. As might be expected for non-excitable tissues, few β subunit transcripts were detected in the zebrafish liver and only zβ1 and zβ2 are expressed in the gill.
Figure 5.
Zebrafish sodium channel β1-4 subunit genes and novel splice variants are differentially expressed in excitable tissues. Total RNA was isolated from wild-type adult zebrafish tissues. RT-PCR with gene and splice variant-specific primers was used to detect expression (see Table 5 for primer sequences and amplicon details). Atr = atrium, Ven = ventricle, Skm = skeletal muscle, Brn = Brain, Eye = eye/optic nerve, Liv = liver, Gil = gill. + = enzyme added to reverse transcription step, - = no reverse transcriptase enzyme (negative control). Zebrafish β-actin was amplified from each template as a positive control.
Table 5.
Primers used to detect expression of zebrafish β subunit genes and splice variants in different tissues of the adult zebrafish.
| Forward Primer (5'-3') | Reverse Primer (5'-3') | Gene | Variant | Amplicon Size (bp) |
| CTACACTTATGCAGAAATGACAGCCAGC | GATGGACAGAGCTTCAAGCTTTTGGCT | zβ1 | A | 422 |
| zβ1 | D | 494 | ||
| GACAGAATCCTCATCTTCCCCAACTATG | CTGCATTCTTCATTTAAACTCAGAGGT | zβ1 | B | 267 |
| zβ1 | D | 534 | ||
| GTCCCTGCGCTGTTGTGTTTAACACAT | GGGTGAACAATCCCTTTAAGCTGCACT | zβ1 | C | 382 |
| CAGCTGACAGACGAGGGCATCTACAACT | CAACACCTGCAGTGAGAAAACCCCATT | zβ2 | A | 201 |
| CATCCTTGCTCTGCTCATTCTGTCCAT | TCGCTACACGATAATACCAGGGAGTGT | zβ2 | B | 304 |
| zβ2 | C | 169 | ||
| zβ2 | D | 236 | ||
| CTGGTGTGTGTGGATGTGCCATCA | CTTGTTGGCGGAAAGCTTGAATGA | zβ3 | - | 357 |
| AGGTGAGCACAGGGAAGGTCCATT | GGAGGCCATTTTCTGTGTTGTCGT | zβ4 | loc1 | 543 |
| TGTGTTGTGTTCATGCTTTG | GACCACCTTTAGTTCCTCTA | zβ4 | loc2 | 395 |
Note: several primer pairs detected multiple splice variants, as indicated above and displayed in Figure 5. BP = base pairs or nucleotide number.
By designing splice variant-specific primers, we also found evidence for tissue-specific regulation of splicing of zebrafish β subunit genes (Fig. 5). While zβ1 variants B and D are expressed primarily in the brain and eye (including the optic nerve), zβ1A is expressed in the atrium, ventricle, skeletal muscle and gill. Similarly, while zβ2 variant B is primarily expressed in the brain and eye, zβ2 variants A, C, and D are also expressed in the atrium, ventricle, brain, eye, and variant D is additionally expressed in the gill. Interestingly, zβ3 may be alternatively-spliced in the brain and eye but not in the atrium or ventricle. We were unable to recover the shorter variant because of its apparent low level of expression. Although zβ1 variant C was identified by RACE-PCR using total embryonic RNA as template, we were unable to detect expression of this short splice variant in the adult tissues analyzed for this study. This suggests that zβ1 variant C may be developmentally regulated, expressed in other tissues, unstable, or is the result of an infrequent alternative splicing event.
Zebrafish β1 functionally modifies sodium channel expression, function in vitro
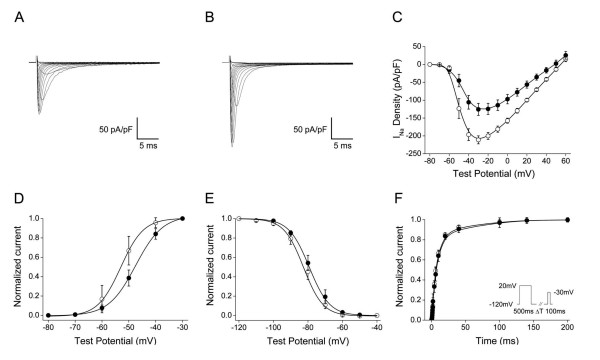
As the first sodium channel auxiliary subunit to be identified, β1 is the most widely-studied of the four known mammalian β subunit proteins. To evaluate whether functional α and β subunit interactions are likely to occur in zebrafish, we heterologously co-expressed the most conserved variant of zβ1 (variant D) with the gene encoding the zebrafish pore-forming sodium channel α subunit zNav1.5, whose expression we detected in the adult zebrafish brain and heart (unpublished observations). Prior studies suggest that the mammalian β1 subunit may influence the current amplitude and possibly the gating of mammalian Nav1.5 in vitro [54-57]. Co-expression of zβ1 with zscn5a in Chinese Hamster Ovary (CHO) cells increased peak sodium current by 68% at a -30 mV depolarizing pulse (n = 5, p < 0.001) compared to zscn5a alone (n = 8) (Fig. 6A–C, table 6). Co-expression of zβ1 with zscn5a also resulted in small but significant changes in the gating of the zNav1.5 channel protein. zβ1 induced hyperpolarizing shifts in both the voltage-dependence of activation and inactivation of zNav1.5, without altering recovery from inactivation (Fig. 6D–F, table 6). These data suggest that the canonical modulatory effects of mammalian sodium channel β subunits on α subunit function and expression are likely to be conserved in teleosts and other non-mammalian vertebrates.
Figure 6.
The zebrafish β1 subunit modulates the biophysical properties of the zebrafish sodium channel α subunit zNav1.5 in CHO cells. A) Typical whole-cell sodium current trace of zNav1.5 following expression of the pBK-CMV-zscn5a expression vector in CHO cells (n = 8). B) Typical whole-cell sodium current trace of zNav1.5 + zβ1 (variant D) following co-expression of pBK-CMV-zscn5a and pGFP-IRES-zβ1D. zβ1 significantly increased the peak amplitude of sodium current by 68% (p = 0.005) at a -30 millivolt (mV) depolarizing pulse (n = 5). C) Current-voltage relationship demonstrating an increase in sodium current at every test potential between -50 and +50 mV. Filled circles = zNav1.5 alone; open circles = zNav1.5 + zβ1. D) Voltage dependence of activation (zNav1.5 alone, n = 8; zNav1.5 + zβ1, n = 5). E) Voltage-dependence of inactivation. (zNav1.5 alone, n = 5; zNav1.5 + zβ1, n = 6). F) Recovery from inactivation (zNav1.5 alone, n = 4; zNav1.5 + zβ1, n = 6). Pulse protocol in inset. Summary data is reported in table 6.
Table 6.
Biophysical properties of zNav1.5 and zNav1.5 plus zβ1 (variant D) in CHO cells.
| Peak amplitude (pA)* | Activation V1/2(mV) | Inactivation V1/2(mV) | Recovery from inactivation, Tau (ms) | |
| zNav1.5 alone | -125.7 ± 16.9 pA/pF (n = 8) | -47.5 ± 1.6 mV (n = 8) | -79.3 ± 1.0 mV (n = 5) | 127.9 ± 8.1 ms (n = 4) |
| zNav1.5 + zβ1 | -211.1 ± 11.2 pA/pF† (n = 5) | -53.0 ± 2.5 mV† (n = 5) | -82.6 ± 1.1 mV† (n = 6) | 125.7 ± 4.1 ms (n = 6) |
All values are mean ± standard error of the mean (SEM). * at a -30 mV depolarizing pulse. † p < 0.001 vs. zNav1.5 alone, Student's T-Test. pA = picoamperes; mV = millivolts; ms = milliseconds; pF = picofahrads; V1/2 = half maximal voltage.
Evolutionary relationships of vertebrate β subunit genes
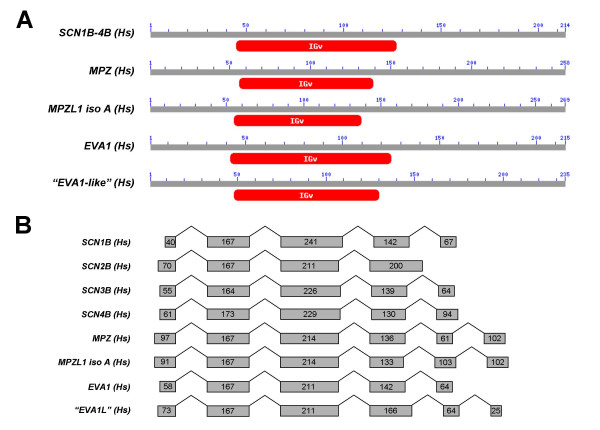
Although β subunit genes appear to be related both structurally and functionally to each other and to cell adhesion molecules with V-type IG domains such as myelin protein zero and contactin [11,12], the evolutionary relationships among these genes have not previously been formally studied. To investigate these relationships, we first identified an extended group of human genes that display sequence homology to β subunits. BLAST searches of the human genome with human β subunit nucleotide and amino acid sequences most frequently-identified the genes myelin protein zero, myelin protein zero-like 1, isoform A (MPZL1 iso A), epithelial V-like antigen (EVA1), and hypothetical protein LOC196264 which we named "EVA1-like gene" (EVA1L) for its similarity to EVA1 and the proximity of these two genes in the human genome. Analysis of the amino acid sequence of each gene using the NCBI conserved domain database and TMpred software revealed that all 4 proteins likely possess single V-type IG domains and transmembrane segments, respectively, similar to sodium channel β subunits (Fig. 7A). Moreover, comparison of the complementary DNA sequences of these genes against their respective genomic loci indicated that all 4 genes also possess genomic organization similar to sodium channel β subunits (Fig. 7B). We therefore included these genes in our analysis of the synteny and phylogeny of the extended family of vertebrate β subunit genes.
Figure 7.
Four additional human genes share homology with β subunits in both sequence and genomic organization. A) BLASTP searches of the human genome using sodium channel β subunit amino acid sequences identified significant homology to myelin P0 protein (MPZ), myelin P0 protein-like protein isoform A (MPZL1 isoform A), epithelial V-like antigen 1 (EVA1), and epithelial V-like antigen 1-like gene (EVA1L), all of which are single transmembrane proteins with extracellular V-type immunoglobulin domains and intracellular C-terminal tails as predicted by NCBI conserved domain database v2.09 and TMPred (see Methods for further details). B) This group of genes additionally shares similar genomic organization. Numbers in grey boxes refer to exon size (nucleotides) with untranslated sequence excluded for clarity.
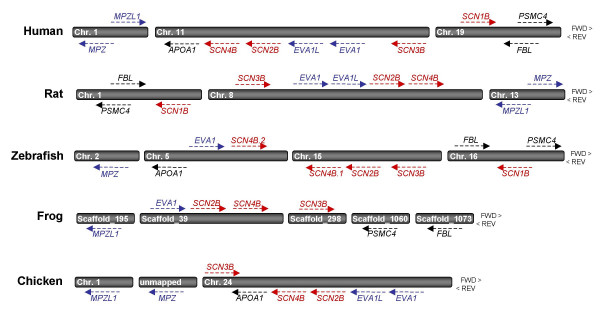
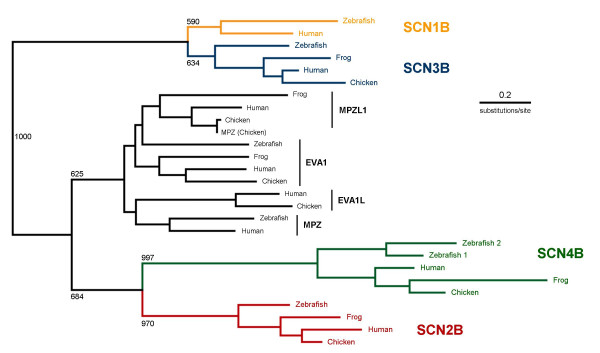
To analyze synteny, we first identified all β subunit-like genes in human, rat, zebrafish, frog (Xenopus tropicalis) and bird (Gallus gallus) genomes (Table 7). Notably, while humans and rats each have 4 and zebrafish have 5 β subunit genes, only 3 β subunit genes were identified in frogs and birds (β1 was not found in either genome). Next, we used reciprocal BLAST searches and in silico chromosome walking to assess physical relationships among β-subunit like genes (Fig. 8). Strikingly, scn2b, scn4b, and EVA1/EVA1L genes map to common locations in every vertebrate genome analyzed (human chromosome 11, rat chromosome 8, zebrafish chromosome 5/15, frog scaffold_39, and chicken chromosome 24), suggestive of a close evolutionary relationship that may have resulted from gene duplication. Although physically more distant, scn3b is also syntenic with this group of genes in 4/5 genomes analyzed. Of the 4 sodium channel β subunits, only scn1b falls outside this syntenic block. The common ancestry of human, rat, and zebrafish scn1b is supported, however, by the proximity of the genes encoding the 26S protease regulatory subunit 6B (PSMC4) and fibrillarin (FBL) to the scn1b locus in all three species (human chromosome 19, rat chromosome 1, zebrafish chromosome 16). The synteny of β subunit genes in mammalian, fish, amphibian, and avian lineages thus supports the hypothesis that extant vertebrate β subunits are orthologous. Moreover, these findings strongly suggest that the evolution of at least several members of this extended gene family (scn2b, scn4b, EVA1, EVA1L) may have arisen from duplication events that predated the divergence of teleosts and tetrapods.
Table 7.
List of cloned and predicted genes utilized for analysis of synteny and phylogeny of the extended β subunit gene family in vertebrates.
| Analysis of Synteny | Analysis of Phylogeny | |||
| Gene | Species | Accession No. (Gene) | Physical Location | Accession No. (Protein) |
| SCN1B | Homo sapiens | ENSG00000105711 | chr. 19 (FWD, 40.21–40.22 Mb) | ENSP00000262631 |
| Rattus norvegicus | ENSRNOG00000021102 | chr. 1 (REV, 86.16–86.17 Mb) | n/a | |
| Danio rerio | ENSDARG00000060222 | chr. 16 (REV, 47.82–47.83 Mb) | ABF47239 | |
| SCN2B | Homo sapiens | ENSG00000149575 | chr. 11 (REV, 117.54–117.55 Mb) | ENSP00000278947 |
| Rattus norvegicus | ENSRNOG00000016221 | chr. 8 (FWD, 48.07–48.08 Mb) | n/a | |
| Danio rerio | ENSDARG00000041176 | chr. 15 (REV, 27.32 Mb) | ABF47241. | |
| Xenopus tropicalis | ENSXETESTG00000009930 | *scaffold_39 (FWD, 0.58–0.59 Mb) | ENSXETESTP00000016971 | |
| Gallus gallus | ENSGALG00000021272 | chr. 24 (REV, 5.10–5.11 Mb) | ENSGALP00000033652 | |
| SCN3B | Homo sapiens | ENSG00000166257 | chr. 11 (REV, 123.01–123.03 Mb) | ENSP00000299333 |
| Rattus norvegicus | ENSRNOG00000006937 | chr. 8 (FWD, 43.23–43.25 Mb) | n/a | |
| Danio rerio | ENSDARESTG00000011553 | chr. 15 (REV, 28.62–28.64 Mb) | ABF47244 | |
| Xenopus tropicalis | ENSXETESTG00000002589 | *scaffold_298 (FWD, 0.94–0.96 Mb) | ENSXETESTP00000004357 | |
| Gallus gallus | ENSGALESTG00000012587 | chr. 24 (FWD, 2.82–2.83 Mb) | ENSGALESTP00000019882 | |
| SCN4B.1 | Homo sapiens | ENSG00000177098 | chr. 11 (REV, 117.51–117.53 Mb) | ENSP00000322460 |
| Rattus norvegicus | ENSRNOG00000026679 | chr. 8 (FWD, 48.09–48.11 Mb) | n/a | |
| Danio rerio | ENSDARESTG00000010162 | chr. 15 (REV, 27.26–27.29 Mb) | ABF47245 | |
| Xenopus tropicalis | ENSXETESTG00000009931 | *scaffold_39 (FWD, 0.61–0.63 Mb) | ENSXETESTP00000016972 | |
| Gallus gallus | ENSGALG00000007409 | chr. 24 (REV, 5.10 Mb) | ENSGALP00000011971 | |
| SCN4B.2 | Danio rerio | ENSDARESTG00000000878 | chr. 5 (FWD, 38.08–38.10 Mb) | ABF47246 |
| MPZ | Homo sapiens | ENSG00000158887 | chr. 1 (REV, 159.54–159.55 Mb) | ENSP00000289928 |
| Rattus norvegicus | ENSRNOESTG00000011377 | chr. 13 (FWD, 87.04–87.05 Mb) | n/a | |
| Danio rerio | ENSDARG00000038609 | chr. 2 (REV, 46.60–46.62 Mb) | ENSDARP00000056371 | |
| Gallus gallus | ENSGALESTG00000015635 | *chr. Un (REV, 60.79–60.80 Mb) | ENSGALP00000008810 | |
| MPZL1 isoA | Homo sapiens | ENSG00000197965 | chr. 1 (FWD, 165.96–166.03 Mb) | ENSP00000352513 |
| Rattus norvegicus | ENSRNOG00000003248 | chr. 13 (REV, 81.32–81.36 Mb) | n/a | |
| Xenopus tropicalis | ENSXETG00000023014 | *scaffold_195 (REV, 1.78–1.79 Mb) | ENSXETP00000049765 | |
| Gallus gallus | ENSGALG00000015438 | chr. 1 (REV, 85.41–85.44 Mb) | ENSGALP00000024855 | |
| EVA1 | Homo sapiens | ENSG00000149573 | chr. 11 (REV, 117.63–117.64 Mb) | ENSP00000278937 |
| Rattus norvegicus | ENSRNOG00000016085 | chr. 8 (FWD, 47.99–48.00 Mb) | n/a | |
| Danio rerio | ENSDARG00000027345 | chr. 5 (FWD, 38.05–38.07 Mb) | ENSDARP00000032601 | |
| Xenopus tropicalis | ENSXETESTG00000008504 | *scaffold_39 (FWD, 0.51–0.54 Mb) | ENSXETESTP00000014524 | |
| Gallus gallus | ENSGALG00000007412 | chr. 24 (REV, 5.12–5.13 Mb) | ENSGALP00000011976 | |
| "EVA1-like" | Homo sapiens | ENSG00000160588 | chr. 11 (REV, 117.60–117.63 Mb) | ENSP00000278949 |
| Rattus norvegicus | ENSRNOG00000026753 | chr. 8 (FWD, 48.00–48.01 Mb) | n/a | |
| Gallus gallus | ENSGALG00000021271 | chr. 24 (REV, 5.11–5.12 Mb) | ENSGALP00000033649 | |
| PSMC4 | Homo sapiens | ENSG00000013275 | chr. 19 (FWD, 45.17–45.18 Mb) | n/a |
| Rattus norvegicus | ENSRNOG00000018994 | chr. 1 (REV, 83.15–83.16 Mb) | n/a | |
| Danio rerio | ENSDARG00000027099 | chr. 16 (FWD, 47.99-5-48.00 Mb) | n/a | |
| Xenopus tropicalis | ENSXETG00000014266 | *scaffold_1060 (REV, 0.17 Mb) | n/a | |
| FBL | Homo sapiens | ENSG00000105202 | chr. 19 (REV, 45.02–45.03 Mb) | n/a |
| Rattus norvegicus | ENSRNOG00000019229 | chr. 1 (FWD, 83.27–83.28 Mb) | n/a | |
| Danio rerio | ENSDARG00000053912 | chr. 16 (FWD, 47.49–47.50 Mb) | n/a | |
| Xenopus tropicalis | ENSXETG00000017830 | *scaffold_1073 (REV, 0.14 Mb) | n/a | |
| APOA1 | Homo sapiens | ENSG00000118137 | chr. 11 (REV, 116.21 Mb) | n/a |
| Danio rerio | ENSDARG00000012076 | chr. 5 (REV, 38.04 Mb) | n/a | |
| Gallus gallus | ENSGALG00000007114 | chr. 24 (REV, 4.79–4.80 Mb) | n/a |
All genes and proteins are identified by Ensembl accession numbers, except for cloned zebrafish beta subunit protein sequences (which begin with ABF and represent accession numbers assigned by GenBank). * = unmapped scaffolds in Ensembl database. FWD = forward and REV = reverse and refers to gene orientation. n/a = not applicable (e.g. proteins for this species were not included in phylogenetic analysis).
Figure 8.
SCN2B, SCN3B and SCN4B are syntenic with each other and with EVA1, EVA-1L, and APOA-1 in multiple vertebrate genomes, while SCN1B is syntenic with FBL and PSMC4 in humans, rats, and zebrafish. To analyze synteny, β subunits and subunit-like genes were identified in human, rat, zebrafish, frog and bird genomes (see Table 7 for gene IDs and physical locations). While humans and rats have four and zebrafish have five β subunit genes, only three β subunit genes were identified in frogs and birds (β1 was not found in either genome). Reciprocal blast searches and in silico chromosome walking were used to assess physical relationships among vertebrate β subunit-like genes. HUGO gene nomenclature symbol IDs: PSMC4 = 26S protease regulatory subunit 6B; FBL = fibrillarin; APOA1 = apolipoprotein A-1; MPZ = myelin protein zero; MPZL1 = myelin protein zero-like gene, isoform A; EVA1 = epithelial V-like antigen 1; EVA1L = unannotated gene similar to EVA1. Red = β subunit genes, Blue = β subunit-like genes, Black = unrelated genes that are syntenic with β subunits.
To further analyze the evolution of vertebrate β subunit-like genes, we reconstructed the phylogeny of this gene family (Fig. 9). Neighbor-joining phylogenetic analysis of amino acid sequences demonstrated that all-identified sodium channel β subunits fall on one of four branches corresponding to β1, β2, β3, or β4, supporting the preliminary identity assigned to each cloned zebrafish β subunit gene (including the duplicated zebrafish β4 genes) by alignment with each individual mammalian subunit (Figs. 1A, 2A, 3A, 4A). Despite synteny among scn2b, scn3b, and scn4b, phylogenetic analysis revealed that β3 is more closely related to β1 than to either β2 or β4. Moreover, our phylogenetic model incorporating β subunit-like genes additionally indicated that β2 and β4 are more closely-related to each other and to β subunit-like genes than to either β1 or β3. Our analysis thus supports an evolutionary model where β2/β4/β subunit-like genes and β1/β3 arose following duplications of 2 distinct precursor genes. The timing of such duplications cannot be gleaned from this model. The presence of gene orthologs to all 4 β subunits in divergent vertebrates (mammals and teleosts) strongly suggests, however, that the early vertebrate common ancestor to these lineages already had four distinct β subunit genes. Duplication events that occurred in this gene family – aside from that which produced two zebrafish β4 genes – thus occurred earlier in vertebrate evolution or prior to the emergence of the vertebrates altogether.
Figure 9.
Phylogenetic analysis demonstrates that vertebrate sodium channel β1-4 subunit genes are orthologous, that β1/β3 and β2/β4 are closely related, and that zβ4.1 and zβ4.2 resulted from a recent gene duplication in fish. Actual (human, zebrafish) and predicted (chicken, frog) amino acid sequences of β subunit and related genes were aligned using CLUSTALX (v1.83). Phylogenetic trees were reconstructed using the neighbor-joining method of Saitou and Nei and viewed with NJPlot software. Alignment gaps were excluded and the Kimura correction was made for multiple substitutions. Bootstrapping (n = 1000) was applied to test the robustness of each node. Tree is unrooted due to the lack of evidence for β subunit-like genes in invertebrate species. HUGO gene nomenclature symbol IDs: MPZ = myelin protein zero; MPZL1 = myelin protein zero-like gene isoform A; EVA1 = epithelial V-like antigen 1; EVA1L = unannotated gene similar to EVA1.
Absence of β subunit genes in invertebrates
The sequenced genomes of the ascidians Ciona intestinalis and Ciona savignyi provide an opportunity to identify putative precursors to vertebrate β subunits genes in invertebrate chordates [58,59]. Although both ascidian genomes contain several loci encoding Nav1 voltage-gated sodium channel α subunits, BLAST searches using the nucleotide and protein sequences of zebrafish and mammalian sodium channel β subunits and related proteins (MPZ, EVA1) did not identify any homologous genes. The absence of β subunit-like genes in sequenced Ciona genomes does not preclude the presence of numerous other genes with V-type and other IG-like domains ([60,61] and unpublished observations). These findings strongly suggest that sodium channel β subunit genes are an innovation of vertebrates. As predicted, searches of the genome of the echinoderm Strongylocentrotus purpuratus (sea urchin) [62], a non-chordate deuterostome, also did not reveal any sodium channel β subunits genes.
Despite the lack of evidence for β subunits genes outside of vertebrates, studies conducted in Drosophila melanogaster demonstrate that invertebrate sodium channels require additional subunits for normal function. Mutations in the Drosophila tip-E (temperature-induced paralysis) gene, which encodes a sodium channel auxiliary subunit, disrupt nerve conduction by perturbing the expression and function of the para voltage-gated sodium channel [63-67]. Moreover, 4 recently-identified tip-E related genes (TEH1-4 or tip-E homologs 1–4) were found to modulate the density and kinetics of sodium currents of heterologously-expressed para sodium channels [68]. Based on these findings, we sought to assess whether vertebrate and invertebrate sodium channel auxiliary subunits share common structural elements or functional domains. Use of TMPred software and the NCBI conserved domain database demonstrated, however, that Drosophila tip-E and tip-E homologous genes (TEH1-4) each possess two predicted membrane-spanning segments (not one) and lack the canonical V-type IG domain found in vertebrate β subunits. Additionally, alignment of the amino acid sequences of cloned zebrafish and human sodium channel β subunit genes with the sequences of Drosophila tip-E and tip-E homologous genes also revealed only minimal homology (< 15% amino acid identity). BLAST searches of the Drosophila genome did not uncover any additional genes sharing close homology with vertebrate β subunits, despite the presence of numerous genes encoding proteins with immunoglobulin domains ([69] and unpublished observations). These results indicate that vertebrate and invertebrate sodium channel auxiliary subunits are dissimilar in structure and unrelated.
Discussion
Numerous studies of mammalian voltage-gated sodium channel β subunits have demonstrated that these small, single membrane-spanning proteins are integral components of sodium channel complexes in excitable tissues, modulators of the expression and function of pore-forming sodium channel α subunits, and candidate genes for clinical disorders linked to perturbed membrane excitability such as arrhythmia and epilepsy [18,70]. Despite the early origins of the Nav1 family of sodium channel α subunits and their cloning from diverse metazoans including eels, jellyfish, flies, and humans, the evolutionary history of sodium channel β subunits has remained obscure. The primary objective of this study was thus to investigate β subunit genes in Danio rerio (zebrafish), a pivotal vertebrate species whose teleost ancestors diverged from mammals over 400 million years ago, in order to gain further insight into the origin and regulation of the voltage-gated sodium channel macromolecular complex.
Using a combination of bioinformatics, molecular cloning, and phylogenetic analysis, we identified conserved orthologs of all 4 mammalian β subunit genes and 8 novel β1 and β2 splice variants in zebrafish. Using our cloned zebrafish sequences, we subsequently identified β subunit genes in other non-mammalian vertebrates including Xenopus tropicalis (Western clawed frog) and Gallus gallus (Red jungle fowl). The existence of conserved mammalian, teleost, amphibian, and avian sodium channel β subunit loci indicates that this gene family is likely to be found in most if not all vertebrate genomes. Moreover, our detection of zebrafish β subunit gene expression in tissues including the heart, muscle, and the brain suggests that the voltage-gated sodium channel α-β subunit macromolecular complex is likely to be a common structural feature of excitable membranes.
Sodium channel complexes in non-mammalian vertebrates: an emerging concept
Our data are the first to support the existence of voltage-gated sodium channel α-β subunit macromolecular complexes in non-mammalian vertebrates. Data from prior studies, however, suggest that sodium channels form α-β subunit complexes in mammals but not in other vertebrate lineages [5-7,71-79]. While sodium channels biochemically purified from rat neuronal membranes and rat and rabbit skeletal muscle membranes were found to be comprised of 1 α subunit and 1 or more auxiliary β subunits, for example, α subunits purified from the electroplax (electric organ) of the South American eel Electrophorus electricus and from chick cardiac muscle were unaccompanied by β subunits [5-7,71-79]. Further investigation into the existence of sodium channel α-β macromolecular complexes in non-mammalian vertebrates is thus needed to reconcile our current findings with the results of previous studies.
There are several possible reasons for the discord between biochemical and molecular genetic approaches. First, it is possible that some but not all voltage-gated sodium channel isoforms within a particular species form complexes by associating with auxiliary β subunits, or that pore-forming α subunits form complexes only in specific tissues. Evidence suggests that even in mammals, for example, the subunit composition of voltage-gated sodium channels significantly varies both by α subunit isoform and by tissue [9,72]. Second, at least for the eel electroplax, the function of this organ may depend on sodium channel α subunits that have evolved unique properties which may preclude association with auxiliary subunits [80]. Third, it is possible that sodium channel α subunit isoforms in certain tissues form complexes not with canonical β subunits but with yet unidentified proteins. In mammals, the interaction of sodium channel α subunits with a number of additional components and modulators in macromolecular complexes suggest that β subunits may not always serve as obligatory functional partners (for review, see refs [18,81]). Our detection of conserved β subunit mRNA expression in excitable organs such as heart, brain, and skeletal muscle, however, suggests the strong possibility of α-β subunit complexes in these zebrafish tissues. Moreover, co-expression of zebrafish α and β subunit genes in CHO cells demonstrated meaningful functional interactions between the two subunits, with significant differences observed in sodium current amplitude and channel gating when both subunit genes are expressed together versus when the α subunit gene is expressed alone. Despite these findings, however, we cannot completely rule out the possibility that sodium channel β subunit genes play different in vivo functional roles in mammals than in non-mammalian vertebrates.
Evolution of sodium channel β subunits and related genes
Our analysis of the synteny and phylogeny of vertebrate sodium channel β subunit genes suggests a different evolutionary history for α and β subunits. For sodium channel α subunits, prevailing evolutionary models posit that the 10 mammalian isoforms arose from the tandem duplication of at least 2 of 4 ancestral sodium channel genes, which in turn had duplicated from 1 or 2 precursor chordate Nav1 sodium channel genes by polyploidization [36-39]. As would be predicted by this model, the total number of Nav1 sodium channel genes varies between teleosts and mammals despite the phylogenetic clustering of all of these genes into 4 groups derived from ancestral vertebrate sodium channel genes [38,40]. Sodium channel β subunits, which are fewer in number, do not appear to have undergone tandem duplication in either mammals or in non-mammalian vertebrates. This is supported by our finding that 4 distinct vertebrate lineages (mammals, ray-finned fishes, amphibians and birds) all appear to share distinct orthologs to 3 or 4 β subunit genes. Thus, it is likely that the common ancestor to teleosts and tetrapods over 400 million years ago also possessed 4 distinct ancestral β subunit genes (β1-4). The presence of 2 β4 orthologs on different zebrafish chromosomes is likely to be the remnant of an additional polyploidization event known to have occurred in teleost vertebrates but not in mammals [41]. It is difficult to determine why duplicate genes for β1-3 have not been retained in zebrafish; it is possible that > 5 β subunit genes did not offer teleosts any evolutionary advantage [42].
The close phylogenetic relationship of SCN1B and SCN3B and the physical proximity of SCN2B and SCN4B to each other and to genes (e.g. EVA1) that are β subunit-like in sequence and genomic organization is strong evidence that duplication events gave rise to the β subunit gene family during the postulated large-scale expansion of the early vertebrate genome. Although we cannot accurately predict the early evolutionary history of sodium channel β subunit genes prior to the divergence of teleosts from other vertebrate lineages, the results of our phylogenetic analysis support the existence of 2 ancestral β subunit genes (β1/β3 and β2/β4) in early vertebrates. Somewhat surprisingly (but in agreement with a previous report [33]), BLASTN and BLASTP searches of available urochordate (Ciona intestinalis, Ciona savignyi) genomes did not identify any sodium channel β subunit-like genes, despite the clear existence of genes encoding proteins with immunoglobulin domains similar to those found in β subunits [60,61]. Moreover, we and others also found limited homology between vertebrate β subunit genes and the tip-E/TEH subunits of the para sodium channel in Drosophila [34,65]. These findings strongly suggest that sodium channel β subunits are a unique vertebrate innovation.
The similarity of vertebrate sodium channel β subunits to members of a more ancient family of IG domain-containing proteins suggests that β subunit genes may have arisen de novo in early vertebrates from genes encoding cell-adhesion molecules, membrane receptors, or components of the innate immunity. The property of modulation of the expression and function of sodium channel β subunits may thus represent a more evolutionarily recent function for this gene family. These findings support the idea that molecular mechanisms enhancing functional diversity and specialization in electrical signaling – either through α subunit gene duplication, editing or alternative splicing of α subunit RNA transcripts, or the emergence of auxiliary proteins that associate with α subunits to modulate their expression and/or function – may have been adaptive and underwent selection in the evolving nervous system of early vertebrates and subsequently, in the increasingly complex excitable tissues of diverse vertebrate lineages including mammals. Despite the lack of common ancestry among invertebrate tip-E/TEH subunits and vertebrate IG-like β subunits, the apparent independent evolution of Nav1 sodium channel-interacting proteins in distantly-related species demonstrates that the formation of sodium channel macromolecular complexes is an evolutionary advantageous and conserved mechanism for fine-tuning the properties of excitable membranes.
Functional implications of alternative splicing of zebrafish β subunit genes
The evidence we present for the extensive C-terminal alternative splicing of zebrafish β subunit genes is intriguing because it may represent another conserved molecular mechanism for modulating electrical signaling in vertebrates. Alternative splicing has been reported for both mammalian sodium channel α and β subunits and invertebrate α subunits, often with important functional consequences [49,50,82-88]. The intronic retention events that produce both the rat β1A and human β1B variants each result in a protein with a novel transmembrane domain and intracellular C-terminal tail. When stably expressed in Chinese Hamster Lung (CHL) fibroblasts, rat β1 and β1A differentially modulate the function of the rat sodium channel α subunit Nav1.2 (scn2a) [49]. Similarly, the human β1 and β1B subunits differentially modulate the function of human Nav1.2 in Xenopus oocytes [50]. These studies suggest that the transmembrane domain and C-terminus of the human and rat β1 subunits contribute to the functional modulation of sodium channel α subunits. In a subsequent study, the β1 intracellular C-terminal tail in particular was found to be required for both efficient physical association with Nav1.2 and for the modulation of sodium channel function in both mammalian cells and Xenopus oocytes [89]. This raises the possibility that the zebrafish β1 and β2 subunit splice variants identified in this study may also differentially influence the function of sodium channel α subunits. Our identification of distinctive expression patterns for different splice variants of the same gene underscores the complexity of this putative sodium channel regulatory mechanism.
Conclusion
We have identified conserved orthologs to all 4 mammalian β subunit genes (zβ1-zβ4) in zebrafish. Despite highly-conserved genomic organization in fish and mammals, zebrafish express 8 distinct mRNA transcripts for the β1 and β2 genes that are generated by alternative splicing. Zebrafish β subunit genes and their splice variants are differentially-expressed in excitable tissues, and co-expression of zβ1 (variant D) with the zebrafish sodium channel α subunit gene zscn5a in CHO cells demonstrated functional α-β interactions that we predict may also occur in native tissues. Our evolutionary analysis of mammalian, teleost, amphibian, and avian β subunit and related genes indicated that all extant vertebrate β subunits are orthologous, that β2/β4 and β1/β3 share common ancestry, and that β subunits are closely-related to other proteins with V-type IG domains including myelin protein zero (MPZ) and epithelial V-like antigen 1 (EVA1). Homologs to vertebrate β subunit genes were not identified in the genomes of invertebrate chordates, and β subunits were found to be unrelated to the tip-E/TEH subunits of the para sodium channel in Drosophila. Taken together, these findings suggest that the family of sodium channel β subunit genes emerged early in vertebrate evolution, prior to the divergence of teleosts and tetrapods. The evolutionary history of vertebrate β subunits is thus consistent with the hypothesis that voltage-gated sodium channel complexes are evolutionarily-conserved structural entities, and that β subunit genes may have played a role in the functional diversification and specialization of electrical signaling in early vertebrates.
Methods
Identification and cloning of zSCN1B-4B genes and splice variants
Mammalian SCN1B-4B gene sequences were used in BLASTN queries of the draft zebrafish genome (Ensembl Zv2-5) to identify DNA contigs containing putative zebrafish orthologs. Primers directed against predicted exons were then used to amplify partial β subunit gene sequences using the Titan RT-PCR enzyme system (Roche) and total day 2 embryonic zebrafish RNA as template. RNA was isolated using Trizol (GIBCO), digested with RQ1 DNase (Promega), and purified with the RNeasy Mini Kit (Qiagen). 5' and 3' ends and alternatively-spliced forms of each gene were identified using 5' and 3' RNA ligase mediated rapid amplification of cDNA ends (RLM-RACE) PCR (Ambion). Additional splice variants were identified by RT-PCR while examining tissue-specific expression. All amplicons were subcloned directly into the pGEM-TEasy vector (Promega) and sequenced in their entirety. Consensus sequences for each gene were established after comparing the forward and reverse sequences of a minimum of 5 clones.
Sequence data and annotation
Eleven novel zebrafish gene sequences were deposited in the NCBI GenBank database: zβ1A [Genbank:DQ489722], zβ1B [Genbank:DQ489723], zβ1C [Genbank:DQ489724], zβ1D [Genbank:DQ489725], zβ2A [Genbank:DQ489726], zβ2B [Genbank:DQ489727], zβ2C [Genbank:DQ489728], zβ2D [Genbank:DQ489729], zβ3 [Genbank:DQ489730], zβ4 locus 1 [Genbank:DQ489731], zβ4 locus 2 [Genbank:DQ489732]. Intron-exon boundaries for each gene and splice variant were determined by comparing cDNA and genomic sequences. Sequences for zebrafish β1-β4 were annotated by alignment with their mammalian orthologs using the AlignX function of Vector NTI v9.1 (Invitrogen) and by use of the following informatics resources: signal peptide (SMART) [90], IG-like domain (SMART [90], NCBI conserved domain database, CDD, v2.06 [91], and REF [14]), transmembrane (TMpred [92] and REF [93]), disulfide bridge (Prosite) [94], and putative N-linked glycosylation sites (NetNGlyc 1.0 server) [95].
Gene expression
Tissues were dissected from wild type adult zebrafish (strain TuAB, 12–16 months old) and flash frozen on dry ice in ethanol. Tissue-specific total RNA was isolated and purified as described above. First strand cDNA synthesis was performed using 1 μg of RNA from each tissue, random hexamer primers, and Transcriptor reverse transcriptase enzyme (Roche). 2 μl of first strand cDNA was used in 25 μl PCR reactions with Expand High Fidelity DNA polymerase (Roche). Amplicons were analyzed on a 1–2% agarose gel made with 1 x Tris-Acetate-EDTA (TAE) buffer. Primer pairs used to amplify each individual gene and splice variant are listed in Table 5. All amplicons were subcloned directly into the pGEM-TEasy vector (Promega) and sequenced in their entirety in both sense and antisense directions.
Identification of β subunit-like genes
Human sodium channel β subunit nucleotide and amino acid sequences were used in BLAST searches of the human genome to identify β subunit-like genes. Four human genes sharing the greatest homology as well as similar genomic organization and/or synteny with known sodium channel β subunit genes were included for further analysis: myelin protein zero (MPZ) [Genbank:NP_000521], myelin protein zero-like gene, isoform A (MPZL1 isoform A) [Genbank:NP_003944], epithelial V-like antigen (EVA) [Genbank:NP_005788], and epithelial V-like antigen-like gene (hypothetical protein LOC196264 or EVA1-like gene) [Genbank:NP_938016].
Analysis of synteny
Synteny between vertebrate β subunits and related genes was assessed by chromosomal walking and reciprocal BLAST searches of genes adjacent to β subunit loci in human, zebrafish, Rattus norvegicus, Xenopus tropicalis, and Gallus gallus genome databases (Ensembl).
Analysis of phylogeny
To estimate phylogeny, additional β subunit and β subunit-like gene sequences were identified in Rattus norvegicus, Xenopus tropicalis, and Gallus gallus genome databases (Ensembl). Predicted amino acid sequences were aligned with cloned zebrafish sequences using CLUSTALX (v1.83). Phylogenetic trees were reconstructed using the neighbor-joining method of Saitou and Nei (Ref [96]) and viewed with NJplot software. Alignment gaps were excluded in the analysis, and the Kimura correction was made for multiple substitutions [97]. The robustness of each node in the phylogenetic tree was analyzed with bootstrap analysis (n = 1000). Trees were unrooted due to limited evidence for β subunit-like genes in non-vertebrate species.
Genome databases
All genome resources utilized for this study were accessed through the Ensembl gateway [98] with the exception of the sea urchin genome, which is available for BLAST searches by the National Human Genome Sequencing Center at Baylor College of Medicine [99].
Expression vectors
The full-length zebrafish β1D splice variant was amplified in one step from total D2 embryonic RNA template using the Titan one-tube RT-PCR system (Roche) and the following primers: Fwd: Spe I-GACTCTGAAAACAAAGCCTG, Rev: Sac II-AGAGCTTCAAGCTTTTGGCT. The 733 base pair product was sublconed directly into the pGEM-TEasy vector. Multiple clones were purified, sequenced, and screened against the consensus sequence determined during the cloning of the zβ1 gene. Insert was digested out of a single zβ1D consensus clone and subcloned into the pGFP-IRES vector (Clontech) using Spe I and Sac II restriction sites. We have used similar methods to identify, clone and assemble a full-length zscn5a expression construct (pBK-CMV-zscn5a) which encodes zebrafish Nav1.5. These methods are described elsewhere (manuscript in preparation).
Transient transfection and electrophysiology
Cultured Chinese Hamster Ovary (CHO) cells were transiently transfected with pBK-CMV-zscn5a and pGFP-IRES-zβ1D constructs using FuGENE6 (Roche). Cells were grown for 48 hours after transfection before electrophysiologic study. Whole-cell voltage clamp was performed at room temperature with 2-MΩ patch microelectrodes and an Axopatch 200A amplifier. To minimize the capacitive transients, we compensated for approximately 70% to 80% of the cell capacitance and series resistance [100]. Cells exhibiting very large currents (> 6 nA) were also excluded from further analysis. Cells were also excluded if voltage control was compromised. The extracellular bath solution contained (in mmol/L) NaCl 145, KCl 4.0, MgCl2 1.0, CaCl2 1.8, glucose 10, and HEPES 10; the pH was 7.4, adjusted with NaOH. The pipette (intracellular) solution contained (in mmol/L) NaF 10, CsF 110, CsCl 20, EGTA 10, and HEPES 10; the pH was 7.4, adjusted with CsOH. Cells were held at -120 mV, and activating currents were elicited with depolarizing pulses from -100 to +50 mV in 10 mV increments. Specific clamp protocols are indicated with the data. Data were acquired by pClamp8.0 (Axon Instruments Inc), sampled at 50 kHz, and low-pass filtered at 5 kHz. All currents were normalized to the cell capacitance calculated by Membrane Test (OUT O) in pClamp8.0.
Abbreviations
BLAST Basic local alignment search tool
BLASTN BLAST nucleotide
BLASTP BLAST protein
dbSNP Database of single nucleotide polymorphisms
HUGO Human genome organization
IRES Internal ribosomal entry site
mRNA Message ribonucleic acid
Nav1 Voltage-gated sodium channel, family 1
NCBI National Center for Biotechnology Information
Para Paralytic gene, Drosophila
PCR Polymerase chain reaction
PharmGKB Pharmacogenomics knowledge base
RACE Rapid amplification of cDNA ends
RT-PCR Reverse transcription polymerase chain reaction
SNPs Single nucleotide polymorphisms
TEH1-4Tip-E homologous genes 1–4
Tip-ETemperature-induced paralysis gene, Drosophila
TMPred Transmembrane prediction software
UTR Untranslated region
zβ1 zebrafish sodium channel beta 1 subunit gene (zebrafish scn1b).
zβ2 zebrafish sodium channel beta 2 subunit gene (zebrafish scn2b).
zβ3 zebrafish sodium channel beta 3 subunit gene (zebrafish scn3b).
zβ4.1 zebrafish sodium channel beta 4.1 subunit gene (zebrafish scn4.1b).
zβ4.2 zebrafish sodium channel beta 4.2 subunit gene (zebrafish scn4.2b).
zscn5a zebrafish homolog to the mammalian sodium channel pore-forming α subunit gene scn5a.
Authors' contributions
SSC designed this study with input from DMR and TPZ. SSC collected, analyzed, and summarized the data for all figures and tables except Figure 6/Table 6, which represent electrophysiological analyses performed by HW using expression constructs built by SSC. DMR and TPZ were the co-principal investigators of this project, overseeing the design, interpretation, and reporting of this study. SSC wrote the manuscript and all authors read and approved of the final version.
Supplementary Material
Hydropathy analysis of zebrafish β subunit amino acid sequences reveals conserved protein secondary structure. TMPred hyropathy plot based on the method of Kyte and Doolittle. Dotted red line drawn at 0 (neutral). SP = signal peptide and TM = transmembrane domain. Dotted line plot (black) indicates outside > inside orientation relative to membrane, while solid line plot (black) indicates inside > outside orientation. A) Hydropathy plots for zβ1 variants A, B, and D are nearly identical (zβ1D shown). B) zβ2A and zβ2B-D. C) zβ3. D) zβ4.1 and zβ4.2.
Prediction of conserved extracellular V-type IG domains in the amino acid sequences of the most highly-conserved splice variants of zβ1-4, NCBI Conserved Domain Database (CDD v2.09. Predicted V-type IG domain is shown in red, and the scale denotes the length of the full-length protein. A) zβ1D, B) zβ2B, C) zβ3, D) zβ4.1, E) zβ4.2.
Acknowledgments
Acknowledgements
This work was supported by Public Health Service award T32 GM07347 from the National Institute of General Medical Studies for the Vanderbilt Medical-Scientist Training Program. Portions of this work were also supported by HL46681 (DMR) and HL073348 (TPZ).
Contributor Information
Sameer S Chopra, Email: sameer.chopra@vanderbilt.edu.
Hiroshi Watanabe, Email: hiroshi.watanabe@vanderbilt.edu.
Tao P Zhong, Email: tao.zhong@vanderbilt.edu.
Dan M Roden, Email: dan.roden@vanderbilt.edu.
References
- Hille B. Ion channels of excitable membranes. 3rd. Sunderland, MA, Sinauer Associates; 2001. [Google Scholar]
- Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4:207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63::871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Anderson PA, Greenberg RM. Phylogeny of ion channels: clues to structure and function. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:17–28. doi: 10.1016/S1096-4959(01)00376-1. [DOI] [PubMed] [Google Scholar]
- Hartshorne RP, Messner DJ, Coppersmith JC, Catterall WA. The saxitoxin receptor of the sodium channel from rat brain. Evidence for two nonidentical beta subunits. J Biol Chem. 1982;257:13888–13891. [PubMed] [Google Scholar]
- Barchi RL. Protein components of the purified sodium channel from rat skeletal muscle sarcolemma. J Neurochem. 1983;40:1377–1385. doi: 10.1111/j.1471-4159.1983.tb13580.x. [DOI] [PubMed] [Google Scholar]
- Roberts RH, Barchi RL. The voltage-sensitive sodium channel from rabbit skeletal muscle. Chemical characterization of subunits. J Biol Chem. 1987;262:2298–2303. [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Catterall WA. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994;12:1183–1194. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation. 2004;109:1421–1427. doi: 10.1161/01.CIR.0000121421.61896.24. [DOI] [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science. 1992;256:839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- Isom LL, Ragsdale DS, De Jongh KS, Westenbroek RE, Reber BF, Scheuer T, Catterall WA. Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell. 1995;83:433–442. doi: 10.1016/0092-8674(95)90121-3. [DOI] [PubMed] [Google Scholar]
- Morgan K, Stevens EB, Shah B, Cox PJ, Dixon AK, Lee K, Pinnock RD, Hughes J, Richardson PJ, Mizuguchi K, Jackson AP. Beta 3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc Natl Acad Sci U S A. 2000;97:2308–2313. doi: 10.1073/pnas.030362197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci. 2003;23:7577–7585. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom LL, Catterall WA. Na+ channel subunits and Ig domains. Nature. 1996;383:307–308. doi: 10.1038/383307b0. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Chen C, Westenbroek RE, Xu X, Edwards CA, Sorenson DR, Chen Y, McEwen DP, O'Malley HA, Bharucha V, Meadows LS, Knudsen GA, Vilaythong A, Noebels JL, Saunders TL, Scheuer T, Shrager P, Catterall WA, Isom LL. Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J Neurosci. 2004;24:4030–4042. doi: 10.1523/JNEUROSCI.4139-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Bharucha V, Chen Y, Westenbroek RE, Brown A, Malhotra JD, Jones D, Avery C, Gillespie PJ, III, Kazen-Gillespie KA, Kazarinova-Noyes K, Shrager P, Saunders TL, Macdonald RL, Ransom BR, Scheuer T, Catterall WA, Isom LL. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel beta 2-subunits. Proc Natl Acad Sci U S A. 2002;99:17072–17077. doi: 10.1073/pnas.212638099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows LS, Isom LL. Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovasc Res. 2005;67:448–458. doi: 10.1016/j.cardiores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Isom LL. Sodium channel beta subunits: anything but auxiliary. Neuroscientist. 2001;7:42–54. doi: 10.1177/107385840100700108. [DOI] [PubMed] [Google Scholar]
- George AL., Jr. Inherited disorders of voltage-gated sodium channels. J Clin Invest. 2005;115:1990–1999. doi: 10.1172/JCI25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, Brice A, LeGuern E, Moulard B, Chaigne D, Buresi C, Malafosse A. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–345. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Tsurubuchi Y, Agarwala KL, Ito M, Fukuma G, Mazaki-Miyazaki E, Nagafuji H, Noda M, Imoto K, Wada K, Mitsudome A, Kaneko S, Montal M, Nagata K, Hirose S, Yamakawa K. A missense mutation of the Na+ channel alpha II subunit gene Na(v)1.2 in a patient with febrile and afebrile seizures causes channel dysfunction. Proc Natl Acad Sci U S A. 2001;98:6384–6389. doi: 10.1073/pnas.111065098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Escayg A, Kearney JA, Trudeau M, MacDonald BT, Mori M, Reichert J, Buxbaum JD, Meisler MH. Sodium channels SCN1A, SCN2A and SCN3A in familial autism. Mol Psychiatry. 2003;8:186–194. doi: 10.1038/sj.mp.4001241. [DOI] [PubMed] [Google Scholar]
- Ptacek LJ, George AL, Jr., Griggs RC, Tawil R, Kallen RG, Barchi RL, Robertson M, Leppert MF. Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell. 1991;67:1021–1027. doi: 10.1016/0092-8674(91)90374-8. [DOI] [PubMed] [Google Scholar]
- Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- Trudeau MM, Dalton JC, Day JW, Ranum LP, Meisler MH. Heterozygosity for a protein truncation mutation of sodium channel SCN8A in a patient with cerebellar atrophy, ataxia, and mental retardation. J Med Genet. 2006;43:527–530. doi: 10.1136/jmg.2005.035667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang Y, Li S, Xu Z, Li H, Ma L, Fan J, Bu D, Liu B, Fan Z, Wu G, Jin J, Ding B, Zhu X, Shen Y. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet. 2004;41:171–174. doi: 10.1136/jmg.2003.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Jr., Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, Berkovic SF, Mulley JC. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19:366–370. doi: 10.1038/448. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Kearney JA, de HG, McEwen DP, Escayg A, Aradi I, MacDonald BT, Levin SI, Soltesz I, Benna P, Montalenti E, Isom LL, Goldin AL, Meisler MH. A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for beta subunit interaction. J Neurosci. 2004;24:10022–10034. doi: 10.1523/JNEUROSCI.2034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An RH, Wang XL, Kerem B, Benhorin J, Medina A, Goldmit M, Kass RS. Novel LQT-3 mutation affects Na+ channel activity through interactions between alpha- and beta1-subunits. Circ Res. 1998;83:141–146. doi: 10.1161/01.res.83.2.141. [DOI] [PubMed] [Google Scholar]
- Wan X, Wang Q, Kirsch GE. Functional suppression of sodium channels by beta(1)-subunits as a molecular mechanism of idiopathic ventricular fibrillation. J Mol Cell Cardiol. 2000;32:1873–1884. doi: 10.1006/jmcc.2000.1223. [DOI] [PubMed] [Google Scholar]
- Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, Ye B, Valdivia C, Ueda K, Canizales-Quinteros S, Tusie-Luna MT, Makielski JC, Ackerman MJ. SCN4B-Encoded Sodium Channel {beta}4 Subunit in Congenital Long-QT Syndrome. Circulation. 2007;116:134–142. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura Y, Nishino A, Murata Y, Nakajo K, Iwasaki H, Ohtsuka Y, Tanaka-Kunishima M, Takahashi N, Hara Y, Yoshida T, Nishida M, Okado H, Watari H, Meinertzhagen IA, Satoh N, Takahashi K, Satou Y, Okada Y, Mori Y. Comprehensive analysis of the ascidian genome reveals novel insights into the molecular evolution of ion channel genes. Physiol Genomics. 2005;22:269–282. doi: 10.1152/physiolgenomics.00229.2004. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/S0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Blackshaw SE, Henderson LP, Malek J, Porter DM, Gross RH, Angstadt JD, Levasseur SM, Maue RA. Single-cell analysis reveals cell-specific patterns of expression of a family of putative voltage-gated sodium channel genes in the leech. J Neurobiol. 2003;55:355–371. doi: 10.1002/neu.10214. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Evolution of voltage-gated Na(+) channels. J Exp Biol. 2002;205:575–584. doi: 10.1242/jeb.205.5.575. [DOI] [PubMed] [Google Scholar]
- Plummer NW, Meisler MH. Evolution and diversity of mammalian sodium channel genes. Genomics. 1999;57:323–331. doi: 10.1006/geno.1998.5735. [DOI] [PubMed] [Google Scholar]
- Lopreato GF, Lu Y, Southwell A, Atkinson NS, Hillis DM, Wilcox TP, Zakon HH. Evolution and divergence of sodium channel genes in vertebrates. Proc Natl Acad Sci U S A. 2001;98:7588–7592. doi: 10.1073/pnas.131171798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkivska H, Hughes AL. Evolution of vertebrate voltage-gated ion channel alpha chains by sequential gene duplication. J Mol Evol. 2003;56:277–285. doi: 10.1007/s00239-002-2399-9. [DOI] [PubMed] [Google Scholar]
- Novak AE, Jost MC, Lu Y, Taylor AD, Zakon HH, Ribera AB. Gene duplications and evolution of vertebrate voltage-gated sodium channels. J Mol Evol. 2006;63:208–221. doi: 10.1007/s00239-005-0287-9. [DOI] [PubMed] [Google Scholar]
- Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- Hughes AL. The evolution of functionally novel proteins after gene duplication. Proc Biol Sci. 1994;256:119–124. doi: 10.1098/rspb.1994.0058. [DOI] [PubMed] [Google Scholar]
- McCormick KA, Isom LL, Ragsdale D, Smith D, Scheuer T, Catterall WA. Molecular determinants of Na+ channel function in the extracellular domain of the beta1 subunit. J Biol Chem. 1998;273:3954–3962. doi: 10.1074/jbc.273.7.3954. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Doyle JP, Hensley P, Colman DR, Hendrickson WA. Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron. 1996;17:435–449. doi: 10.1016/S0896-6273(00)80176-2. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Koopmann MC, Kazen-Gillespie KA, Fettman N, Hortsch M, Isom LL. Structural requirements for interaction of sodium channel beta 1 subunits with ankyrin. J Biol Chem. 2002;277:26681–26688. doi: 10.1074/jbc.M202354200. [DOI] [PubMed] [Google Scholar]
- Kim DY, Ingano LA, Carey BW, Pettingell WH, Kovacs DM. Presenilin/gamma-secretase-mediated cleavage of the voltage-gated sodium channel beta2-subunit regulates cell adhesion and migration. J Biol Chem. 2005;280:23251–23261. doi: 10.1074/jbc.M412938200. [DOI] [PubMed] [Google Scholar]
- Scheffer IE, Harkin LA, Grinton BE, Dibbens LM, Turner SJ, Zielinski MA, Xu R, Jackson G, Adams J, Connellan M, Petrou S, Wellard RM, Briellmann RS, Wallace RH, Mulley JC, Berkovic SF. Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain. 2007;130(Pt.1):100–109. doi: 10.1093/brain/awl272. [DOI] [PubMed] [Google Scholar]
- Audenaert D, Claes L, Ceulemans B, Lofgren A, Van BC, De JP. A deletion in SCN1B is associated with febrile seizures and early-onset absence epilepsy. Neurology. 2003;61:854–856. doi: 10.1212/01.wnl.0000080362.55784.1c. [DOI] [PubMed] [Google Scholar]
- Kazen-Gillespie KA, Ragsdale DS, D'Andrea MR, Mattei LN, Rogers KE, Isom LL. Cloning, localization, and functional expression of sodium channel beta1A subunits. J Biol Chem. 2000;275:1079–1088. doi: 10.1074/jbc.275.2.1079. [DOI] [PubMed] [Google Scholar]
- Qin N, D'Andrea MR, Lubin ML, Shafaee N, Codd EE, Correa AM. Molecular cloning and functional expression of the human sodium channel beta1B subunit, a novel splicing variant of the beta1 subunit. Eur J Biochem. 2003;270:4762–4770. doi: 10.1046/j.1432-1033.2003.03878.x. [DOI] [PubMed] [Google Scholar]
- Oh Y, Waxman SG. The beta 1 subunit mRNA of the rat brain Na+ channel is expressed in glial cells. Proc Natl Acad Sci U S A. 1994;91:9985–9989. doi: 10.1073/pnas.91.21.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Waxman SG. Genes encoding the beta 1 subunit of voltage-dependent Na+ channel in rat, mouse and human contain conserved introns. FEBS Lett. 1995;377:485–488. doi: 10.1016/0014-5793(95)01400-4. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kazen-Gillespie K, Hortsch M, Isom LL. Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J Biol Chem. 2000;275:11383–11388. doi: 10.1074/jbc.275.15.11383. [DOI] [PubMed] [Google Scholar]
- Makita N, Bennett PB, Jr., George AL., Jr. Voltage-gated Na+ channel beta 1 subunit mRNA expressed in adult human skeletal muscle, heart, and brain is encoded by a single gene. J Biol Chem. 1994;269:7571–7578. [PubMed] [Google Scholar]
- Qu Y, Isom LL, Westenbroek RE, Rogers JC, Tanada TN, McCormick KA, Scheuer T, Catterall WA. Modulation of cardiac Na+ channel expression in Xenopus oocytes by beta 1 subunits. J Biol Chem. 1995;270:25696–25701. doi: 10.1074/jbc.270.43.25696. [DOI] [PubMed] [Google Scholar]
- Nuss HB, Chiamvimonvat N, Perez-Garcia MT, Tomaselli GF, Marban E. Functional association of the beta 1 subunit with human cardiac (hH1) and rat skeletal muscle (mu 1) sodium channel alpha subunits expressed in Xenopus oocytes. J Gen Physiol. 1995;106:1171–1191. doi: 10.1085/jgp.106.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YF, Wright SN, Wang GK, Morgan JP, Leaf A. Coexpression with beta(1)-subunit modifies the kinetics and fatty acid block of hH1(alpha) Na(+) channels. Am J Physiol Heart Circ Physiol. 2000;279:H35–H46. doi: 10.1152/ajpheart.2000.279.1.H35. [DOI] [PubMed] [Google Scholar]
- Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De TA, Davidson B, Di GA, Gelpke M, Goodstein DM, Harafuji N, Hastings KE, Ho I, Hotta K, Huang W, Kawashima T, Lemaire P, Martinez D, Meinertzhagen IA, Necula S, Nonaka M, Putnam N, Rash S, Saiga H, Satake M, Terry A, Yamada L, Wang HG, Awazu S, Azumi K, Boore J, Branno M, Chin-Bow S, DeSantis R, Doyle S, Francino P, Keys DN, Haga S, Hayashi H, Hino K, Imai KS, Inaba K, Kano S, Kobayashi K, Kobayashi M, Lee BI, Makabe KW, Manohar C, Matassi G, Medina M, Mochizuki Y, Mount S, Morishita T, Miura S, Nakayama A, Nishizaka S, Nomoto H, Ohta F, Oishi K, Rigoutsos I, Sano M, Sasaki A, Sasakura Y, Shoguchi E, T S, Spagnuolo A, Stainier D, Suzuki MM, Tassy O, Takatori N, Tokuoka M, Yagi K, Yoshizaki F, Wada S, Zhang C, Hyatt PD, Larimer F, Detter C, Doggett N, Glavina T, Hawkins T, Richardson P, Lucas S, Kohara Y, Levine M, Satoh N, Rokhsar DS. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- Canestro C, Bassham S, Postlethwait JH. Seeing chordate evolution through the Ciona genome sequence. Genome Biol. 2003;4:208. doi: 10.1186/gb-2003-4-3-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azumi K, De SR, De TA, Rigoutsos I, Yoshizaki F, Pinto MR, Marino R, Shida K, Ikeda M, Ikeda M, Arai M, Inoue Y, Shimizu T, Satoh N, Rokhsar DS, Du PL, Kasahara M, Satake M, Nonaka M. Genomic analysis of immunity in a Urochordate and the emergence of the vertebrate immune system: "waiting for Godot". Immunogenetics. 2003;55:570–581. doi: 10.1007/s00251-003-0606-5. [DOI] [PubMed] [Google Scholar]
- Du PL, Zucchetti I, De SR. Immunoglobulin superfamily receptors in protochordates: before RAG time. Immunol Rev. 2004;198:233–248. doi: 10.1111/j.0105-2896.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, Angerer RC, Angerer LM, Arnone MI, Burgess DR, Burke RD, Coffman JA, Dean M, Elphick MR, Ettensohn CA, Foltz KR, Hamdoun A, Hynes RO, Klein WH, Marzluff W, McClay DR, Morris RL, Mushegian A, Rast JP, Smith LC, Thorndyke MC, Vacquier VD, Wessel GM, Wray G, Zhang L, Elsik CG, Ermolaeva O, Hlavina W, Hofmann G, Kitts P, Landrum MJ, Mackey AJ, Maglott D, Panopoulou G, Poustka AJ, Pruitt K, Sapojnikov V, Song X, Souvorov A, Solovyev V, Wei Z, Whittaker CA, Worley K, Durbin KJ, Shen Y, Fedrigo O, Garfield D, Haygood R, Primus A, Satija R, Severson T, Gonzalez-Garay ML, Jackson AR, Milosavljevic A, Tong M, Killian CE, Livingston BT, Wilt FH, Adams N, Belle R, Carbonneau S, Cheung R, Cormier P, Cosson B, Croce J, Fernandez-Guerra A, Geneviere AM, Goel M, Kelkar H, Morales J, Mulner-Lorillon O, Robertson AJ, Goldstone JV, Cole B, Epel D, Gold B, Hahn ME, Howard-Ashby M, Scally M, Stegeman JJ, Allgood EL, Cool J, Judkins KM, McCafferty SS, Musante AM, Obar RA, Rawson AP, Rossetti BJ, Gibbons IR, Hoffman MP, Leone A, Istrail S, Materna SC, Samanta MP, Stolc V, Tongprasit W, Tu Q, Bergeron KF, Brandhorst BP, Whittle J, Berney K, Bottjer DJ, Calestani C, Peterson K, Chow E, Yuan QA, Elhaik E, Graur D, Reese JT, Bosdet I, Heesun S, Marra MA, Schein J, Anderson MK, Brockton V, Buckley KM, Cohen AH, Fugmann SD, Hibino T, Loza-Coll M, Majeske AJ, Messier C, Nair SV, Pancer Z, Terwilliger DP, Agca C, Arboleda E, Chen N, Churcher AM, Hallbook F, Humphrey GW, Idris MM, Kiyama T, Liang S, Mellott D, Mu X, Murray G, Olinski RP, Raible F, Rowe M, Taylor JS, Tessmar-Raible K, Wang D, Wilson KH, Yaguchi S, Gaasterland T, Galindo BE, Gunaratne HJ, Juliano C, Kinukawa M, Moy GW, Neill AT, Nomura M, Raisch M, Reade A, Roux MM, Song JL, Su YH, Townley IK, Voronina E, Wong JL, Amore G, Branno M, Brown ER, Cavalieri V, Duboc V, Duloquin L, Flytzanis C, Gache C, Lapraz F, Lepage T, Locascio A, Martinez P, Matassi G, Matranga V, Range R, Rizzo F, Rottinger E, Beane W, Bradham C, Byrum C, Glenn T, Hussain S, Manning G, Miranda E, Thomason R, Walton K, Wikramanayke A, Wu SY, Xu R, Brown CT, Chen L, Gray RF, Lee PY, Nam J, Oliveri P, Smith J, Muzny D, Bell S, Chacko J, Cree A, Curry S, Davis C, Dinh H, Dugan-Rocha S, Fowler J, Gill R, Hamilton C, Hernandez J, Hines S, Hume J, Jackson L, Jolivet A, Kovar C, Lee S, Lewis L, Miner G, Morgan M, Nazareth LV, Okwuonu G, Parker D, Pu LL, Thorn R, Wright R. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314:941–952. doi: 10.1126/science.1133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson FR, Wilson SD, Hall LM. The tip-E mutation of Drosophila decreases saxitoxin binding and interacts with other mutations affecting nerve membrane excitability. J Neurogenet. 1986;3:1–17. doi: 10.3109/01677068609106891. [DOI] [PubMed] [Google Scholar]
- O'Dowd DK, Aldrich RW. Voltage-clamp analysis of sodium channels in wild-type and mutant Drosophila neurons. J Neurosci. 1988;8:3633–3643. doi: 10.1523/JNEUROSCI.08-10-03633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Deak P, Chopra M, Hall LM. Cloning and functional analysis of TipE, a novel membrane protein that enhances Drosophila para sodium channel function. Cell. 1995;82:1001–1011. doi: 10.1016/0092-8674(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Warmke JW, Reenan RA, Wang P, Qian S, Arena JP, Wang J, Wunderler D, Liu K, Kaczorowski GJ, Van der Ploeg LH, Ganetzky B, Cohen CJ. Functional expression of Drosophila para sodium channels. Modulation by the membrane protein TipE and toxin pharmacology. J Gen Physiol. 1997;110:119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges DD, Lee D, Preston CF, Boswell K, Hall LM, O'Dowd DK. tipE regulates Na+-dependent repetitive firing in Drosophila neurons. Mol Cell Neurosci. 2002;19:402–416. doi: 10.1006/mcne.2001.1088. [DOI] [PubMed] [Google Scholar]
- Derst C, Walther C, Veh RW, Wicher D, Heinemann SH. Four novel sequences in Drosophila melanogaster homologous to the auxiliary Para sodium channel subunit TipE. Biochem Biophys Res Commun. 2006;339:939–948. doi: 10.1016/j.bbrc.2005.11.096. [DOI] [PubMed] [Google Scholar]
- Hobert O, Hutter H, Hynes RO. The immunoglobulin superfamily in Caenorhabditis elegans and Drosophila melanogaster. Development. 2004;131:2237–2238. doi: 10.1242/dev.01183. [DOI] [PubMed] [Google Scholar]
- Hartshorne RP, Catterall WA. Purification of the saxitoxin receptor of the sodium channel from rat brain. Proc Natl Acad Sci U S A. 1981;78:4620–4624. doi: 10.1073/pnas.78.7.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne RP, Catterall WA. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984;259:1667–1675. [PubMed] [Google Scholar]
- Hartshorne RP, Keller BU, Talvenheimo JA, Catterall WA, Montal M. Functional reconstitution of the purified brain sodium channel in planar lipid bilayers. Proc Natl Acad Sci U S A. 1985;82:240–244. doi: 10.1073/pnas.82.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew WS, Levinson SR, Brabson JS, Raftery MA. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci U S A. 1978;75:2606–2610. doi: 10.1073/pnas.75.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Agnew WS, Levinson SR. Principal glycopeptide of the tetrodotoxin/saxitoxin binding protein from Electrophorus electricus: isolation and partial chemical and physical characterization. Biochemistry. 1983;22:462–470. doi: 10.1021/bi00271a032. [DOI] [PubMed] [Google Scholar]
- Rosenberg RL, Tomiko SA, Agnew WS. Single-channel properties of the reconstituted voltage-regulated Na channel isolated from the electroplax of Electrophorus electricus. Proc Natl Acad Sci U S A. 1984;81:5594–5598. doi: 10.1073/pnas.81.17.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio-Pinto E, Duch DS, Levinson SR, Urban BW. Purified and unpurified sodium channels from eel electroplax in planar lipid bilayers. J Gen Physiol. 1987;90:375–395. doi: 10.1085/jgp.90.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadei JM, Gordon RD, Barchi RL. Immunoaffinity isolation of Na+ channels from rat skeletal muscle. Analysis of subunits. J Biol Chem. 1986;261:4318–4323. [PubMed] [Google Scholar]
- Lombet A, Lazdunski M. Characterization, solubilization, affinity labeling and purification of the cardiac Na+ channel using Tityus toxin gamma. Eur J Biochem. 1984;141:651–660. doi: 10.1111/j.1432-1033.1984.tb08241.x. [DOI] [PubMed] [Google Scholar]
- Zakon HH, Lu Y, Zwickl DJ, Hillis DM. Sodium channel genes and the evolution of diversity in communication signals of electric fishes: Convergent molecular evolution. Proc Natl Acad Sci U S A. 2006;103:3675–3680. doi: 10.1073/pnas.0600160103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abriel H, Kass RS. Regulation of the voltage-gated cardiac sodium channel Nav1.5 by interacting proteins. Trends Cardiovasc Med. 2005;15:35–40. doi: 10.1016/j.tcm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Camacho JA, Hensellek S, Rougier JS, Blechschmidt S, Abriel H, Benndorf K, Zimmer T. Modulation of Nav1.5 channel function by an alternatively spliced sequence in the DII/DIII linker region. J Biol Chem. 2006;281:9498–9506. doi: 10.1074/jbc.M509716200. [DOI] [PubMed] [Google Scholar]
- Thimmapaya R, Neelands T, Niforatos W, vis-Taber RA, Choi W, Putman CB, Kroeger PE, Packer J, Gopalakrishnan M, Faltynek CR, Surowy CS, Scott VE. Distribution and functional characterization of human Nav1.3 splice variants. Eur J Neurosci. 2005;22:1–9. doi: 10.1111/j.1460-9568.2005.04155.x. [DOI] [PubMed] [Google Scholar]
- Tan BH, Valdivia CR, Rok BA, Ye B, Ruwaldt KM, Tester DJ, Ackerman MJ, Makielski JC. Common human SCN5A polymorphisms have altered electrophysiology when expressed in Q1077 splice variants. Heart Rhythm. 2005;2:741–747. doi: 10.1016/j.hrthm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Makielski JC, Ye B, Valdivia CR, Pagel MD, Pu J, Tester DJ, Ackerman MJ. A ubiquitous splice variant and a common polymorphism affect heterologous expression of recombinant human SCN5A heart sodium channels. Circ Res. 2003;93:821–828. doi: 10.1161/01.RES.0000096652.14509.96. [DOI] [PubMed] [Google Scholar]
- Zimmer T, Bollensdorff C, Haufe V, Birch-Hirschfeld E, Benndorf K. Mouse heart Na+ channels: primary structure and function of two isoforms and alternatively spliced variants. Am J Physiol Heart Circ Physiol. 2002;282:H1007–H1017. doi: 10.1152/ajpheart.00644.2001. [DOI] [PubMed] [Google Scholar]
- Dietrich PS, McGivern JG, Delgado SG, Koch BD, Eglen RM, Hunter JC, Sangameswaran L. Functional analysis of a voltage-gated sodium channel and its splice variant from rat dorsal root ganglia. J Neurochem. 1998;70:2262–2272. doi: 10.1046/j.1471-4159.1998.70062262.x. [DOI] [PubMed] [Google Scholar]
- O'Dowd DK, Gee JR, Smith MA. Sodium current density correlates with expression of specific alternatively spliced sodium channel mRNAs in single neurons. J Neurosci. 1995;15:4005–4012. doi: 10.1523/JNEUROSCI.15-05-04005.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows L, Malhotra JD, Stetzer A, Isom LL, Ragsdale DS. The intracellular segment of the sodium channel beta 1 subunit is required for its efficient association with the channel alpha subunit. J Neurochem. 2001;76:1871–1878. doi: 10.1046/j.1471-4159.2001.00192.x. [DOI] [PubMed] [Google Scholar]
- Simple Modular Architecture Research Tool (SMART) [http://smart.embl-heidelberg.de/] 2007. [DOI] [PMC free article] [PubMed]
- Conserved Domain Database, NCBI [http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi] 2007.
- TM Pred [http://www.ch.embnet.org/] 2007.
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Prosite [http://ca.expasy.org/] 2007.
- NetNGlyc [http://www.cbs.dtu.dk/services/] 2007.
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Kimura M. The Neutral Theory of Molecular Evolution. Cambridge, Cambridge University Press; 1983. [Google Scholar]
- Ensembl genome browser [www.ensembl.org] 2007.
- National Human Genome Sequencing Center, Baylor College of Medicine [http://www.hgsc.bcm.tmc.edu/projects/seaurchin/]. 2007.
- Darbar D, Yang T, Churchwell K, Wilde AA, Roden DM. Unmasking of brugada syndrome by lithium. Circulation. 2005;112:1527–1531. doi: 10.1161/CIRCULATIONAHA.105.548487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubanks J, Srinivasan J, Dinulos MB, Disteche CM, Catterall WA. Structure and chromosomal localization of the beta2 subunit of the human brain sodium channel. Neuroreport. 1997;8:2775–2779. doi: 10.1097/00001756-199708180-00025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hydropathy analysis of zebrafish β subunit amino acid sequences reveals conserved protein secondary structure. TMPred hyropathy plot based on the method of Kyte and Doolittle. Dotted red line drawn at 0 (neutral). SP = signal peptide and TM = transmembrane domain. Dotted line plot (black) indicates outside > inside orientation relative to membrane, while solid line plot (black) indicates inside > outside orientation. A) Hydropathy plots for zβ1 variants A, B, and D are nearly identical (zβ1D shown). B) zβ2A and zβ2B-D. C) zβ3. D) zβ4.1 and zβ4.2.
Prediction of conserved extracellular V-type IG domains in the amino acid sequences of the most highly-conserved splice variants of zβ1-4, NCBI Conserved Domain Database (CDD v2.09. Predicted V-type IG domain is shown in red, and the scale denotes the length of the full-length protein. A) zβ1D, B) zβ2B, C) zβ3, D) zβ4.1, E) zβ4.2.