Summary
Conjunctival mucosa-associated lymphoid tissue (MALT) lymphoma is an extranodal marginal zone B-cell lymphoma that is characterized by an exaggerated clonal expansion of B cells, which implicate a pathological proliferative response to antigen(s) including bacteria. Helicobacter pylori (H. pylori) infection is recognized as one of the causative agents of gastric MALT lymphoma; however, it has not been reported in extra gastric MALT lymphoma. We studied 5 patients (4 adults and 1 child) with salmon-colored conjunctival lesions. One patient also had a history of abnormal bone marrow biopsy a year earlier with lymphoid aggregates involving 5% of the overall bone marrow. The conjunctival lesions of the 5 patients were biopsied. Histopathological diagnoses were consistent with conjunctival MALT lymphoma. Lymphoma and normal conjunctival cells were microdissected using laser capture microscopy or manual techniques. DNA was extracted and subjected to PCR amplification using H. pylori gene-specific primers from the urease B and vac/m2 gene. Cells from chronic conjunctivitis (normal lymphocytes), conjunctival human T-cell lymphotropic virus type-1/adult T-cell leukemia/lymphoma (HTLV-1/ATL), and orbital B-cell lymphoma were also microdissected, processed and analyzed. PCR amplification and Southern blot hybridization demonstrated H. pylori DNA in the conjunctival MALT lymphoma cells of 4/5 cases. The negative case was the one with a history of abnormal bone marrow. In contrast, H. pylori gene was not detected in normal conjunctival cells from the cases of MALT lymphoma or the lymphocytes, ATL and orbital B-lymphoma cells from the controls. These data suggest that H. pylori may play a role in conjunctival MALT lymphoma.
Keywords: Mucosa-associated lymphoid tissue (MALT) lymphoma, Helicobacter pylori (H. pylori), Conjunctiva, microdissection, PCR
Introduction
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is listed as a distinct clinico-pathologic entity in the recently published WHO classification of malignant lymphomas (Isaacson et al., 2001). The majority of MALT lymphomas occur in the gastrointestinal organs, but this type of lymphoma may affect virtually every organ including the ocular adnexa, lung, liver, breast, salivary glands, thyroid, thymus, and skin ( Thieblemont et al., 1995 ; Zinzani et al., 1999 ). MALT lymphomas often remain localized to their sites of origin for many years prior to dissemination and therefore an indolent clinical course is common ( Thieblemont et al., 2000 ). Histologically, MALT lymphomas are characterized by heterogeneous cell proliferation consisting of centrocyte-like, monocytoid, and plasmacytoid tumor cells with occasional blasts in the marginal zone surrounding reactive follicles ( Harris and Isaacson, 1999 ; Isaacson and Wright, 1983 ). Another characteristic feature is secondary infiltration of the germinal centers by neoplastic marginal zone B-cells. When arising in tissues associated with epithelium, nests of MALT lymphoma cells may infiltrate the epithelial structures to generate lymphoepithelial lesions and colonize reactive lymphoid follicles ( Isaacson et al., 2001 ; Coupland et al., 2002 ). The pathogenesis of MALT lymphoma is unknown, but antigen-stimulated B-cells in tissues with epithelial cells in close relationship with germinal centers may be a key factor ( Harris and Isaacson, 1999 ; Wotherspoon et al., 2002 ).
Helicobacter pylori (H. pylori) infection is responsible for gastric and duodenal ulcers, gastric carcinoma, and gastric MALT lymphoma ( Cavalli et al., 2001 ; Marshall, 2002 ; Suerbaum and Michetti, 2002 ). It also significantly increases the risk of gastric MALT lymphoma; 72-98% of patients with gastric MALT lymphoma are infected with H. pylori ( Parsonnet et al., 1994 ). Tumor-infiltrating T cells specifically activated by H. pylori have been documented in vitro in some cases of gastric MALT lymphoma ( Hussell et al., 1996 ). Furthermore, eradication of H. pylori alone induces regression of gastric MALT lymphoma in 70-80% of cases ( Bayerdorffer et al., 1995 , 1997 ; Neubauer et al., 1997 ). Resistance of lymphoma to eradiation therapy is strongly associated with certain genetic abnormalities in the host, such as the translocation t(11;18)(q21;q21), and is often associated with progression to high-grade malignancy ( Liu et al., 2002 ). Most of the patients whose lymphomas respond to eradication therapy stay in remission for several years ( Neubauer et al., 1997 ).
Outside the stomach the role of infectious agents in MALT lymphoma is less clearly defined, although there is a documented association of H. pylori and some autoimmune disorders such as Sjögren syndrome, Henoch-Schonlein purpura, chronic idiopathic urticaria, rosacea, autoimmune thyroiditis and even ischemic heart disease ( Gasbarrini and Franceschi, 1999 ). Salivary gland MALT lymphoma was reported in one elderly Japanese lady with Sjögren syndrome ( Nishimura et al., 2000 ) and there is some evidence that Borrelia burgdorferi may be implicated in the pathogenesis of some cases of cutaneous lymphoma ( Roggero et al., 2000 ). To date, no infectious agents including H. pylori have been associated with conjunctival MALT lymphoma. Here, we seek to identify H. pylori genes in 5 cases of conjunctival MALT lymphoma from formalin fixed, paraffin embedded sections.
Materials and methods
Cases
Conjunctival biopsies of fleshy, salmon-colored lesions were performed in 5 Caucasian patients, ages 10, 58, 61, 71, and 80 years from 1999 to 2002. The biopsy from the child and the slides of the 4 adult biopsies were submitted to the National Eye Institute, USA.
All patients presented with slow growing, painless conjunctival lesions in one eye except the child who had lesions in both eyes. The reminder of the ophthalmic examination was normal. A complete physical examination including lymphoma staging and a bone marrow biopsy was also performed and found to be normal except in one case (#3, 61 year-old female patient), who had a history of abnormal bone marrow biopsy in the previous year which showed lymphoid aggregates involving 5% or less of the overall bone marrow. However, there was no evidence of lymphoma including gastric MALT lymphoma elsewhere. Serum antibodies against H. pylori were evaluated in only the first two patients and were negative for the young child (Case 1) but positive for the adult patient (Case 2, the 58 year-old with H. pylori gastritis who was treated with antibiotics). Rapid urease test for H. pylori was not performed. All adult patients received radiation therapy and all had complete remission with a minimum of one-year follow-up ( Sharara et al., 2003 ). However, the child who did not receive local radiation and chemotherapy developed bilateral recurrent conjunctival lesions 6 months after the biopsy.
The lesions obtained from the 5 patients were submitted for histology, immunohistochemistry, and molecular pathology. All cases were fixed in 10% buffered formalin and one case (the child) also had a portion snap frozen in optical cutting medium. Serial sections were collected and stained with hemotoxylin and eosin as well as immunohistochemistry using the avidin-biotin-complex immunoperoxidase technique. The primary antibodies included monoclonal anti-human CD3, CD4, CD20, CD68, kappa and lambda.
Microdissection
The cells in the lesion (lymphoma cells) and away from the lesion (normal conjunctival cells) were carefully microdissected as previously described (Shen et al., 1998,2001). Briefly, either frozen or 10% buffered formalin fixed-paraffin sections were stained with hematoxylin and eosin. Paraffin sections required deparaffinization. Cells of interest were selected by visualization under the light microscope and microdissected by a 30-gauge needle or by laser capture microscopy, PixCell IITM (Arcturus, Mountain View, CA). The PixCell IITM uses a low power infrared laser to collect selected cells onto a membrane located on the cap of a 1.5 ml tube. In manual microdissection the selected cells were gently scraped, detached and removed from the slide using a 30-gauge needle.
Detection of immunoglobulin gene rearrangements
The microdissected cells in the lesions were immediately placed in proteinase K enriched DNA extraction buffer. PCR amplification was performed with a mixture of 1 μl extracted DNA, 3.0 pmol of 32P-labeled sense primer, 3.0 pmol antisense primer, 4.0 nmol of each dNTP, 1xGeneAmp buffer, 1.0 U of AmpliTag Gold Polymerase (Perkin Elmer, Hayward, CA) and 1.5 mM MgCl2. PCR cycle conditions included a hot start at 94 °C for 9 min, 40 cycles of denaturing at 94 °C for 45 sec, annealing at 58 °C (for immunoglobulin heavy chain (IgH) primers) and extension at 72 °C for 120 sec. IgH FR3A primer (sense: 5’- ACA CGG CYS TGT ATT ACT GT -3’, and antisense 5’- CGA TGG TAC CAA GCT TTG AGG AGA CGG TGA CCA -3’) was chosen for detection of B cell monoclonality and malignancy.
Molecular detection of H. pylori gene
DNA isolated from the microdissected cells from within and outside the lesions also underwent PCR amplification using H. pylori gene-specific primers of HPU54 and HPU18 from the urease B gene ( Clayton et al., 1992 ). Primers HPU54 (5’-TGG GAT TAG CGA GTA TAT-3’) and HPU18 (5’-CCC ATT TGA CTC AAT G-3’) amplified a 132 bp product from the urease gene B (nt 1971 to 2102). The running conditions were hot start at 94 °C for 9 min, 40 cycles of denaturing at 94 °C for 45 sec, annealing for 60 sec at 47 °C, and extension at 72 °C for 120 sec. The reaction was completed after incubation at 72 °C for 10 min. Electrophoresis on a 15% polyacrylamide ethidium bromide-stained gel was employed for separation and visualization of the PCR products with radioisotope labeled primers.
The m2 region of H. pylori vacA gene (vacA m2) was amplified by nested PCR as described by Odenthal and colleagues ( Koehler et al., 2003 ). The primer sets for the first PCR were m2F1 (5’-TTT GGA GC(C/T) CCA GGA AAC ATT G-3’) and m2R1 (5’-C(C/T)A CAC GCC CAT CTT GGA CAA-3’), and for the second PCR were P-32 labeled m2F2 (5’-ACC CTA AA(C/T) AGC AAC GCA AGC-3’) and m2R2 (5’-GAC AAA AAG ATT CAT CGT GCC TT-3’). PCR was performed with a hot start at 94 °C for 9 min, followed by 35 cycles of denaturing at 94 °C for 45 sec, annealing for 60 sec at 57 °C, and extension at 72 °C for 120 sec for the first PCR, and 42 cycles of denaturing at 94 °C for 45 sec, annealing for 60 sec at 58 °C, and extension at 72 °C for 120 sec for the second PCR. One μl DNA template from the first PCR was used for the second amplification. The 101 bp product of the nested PCR amplification was visualized by polyacrylamide gel electrophoresis and autoradiography with radioisotope labeled primers.
Southern hybridization was performed using the probe with a sequence of 5’-GAA CCC GCC TTT GAT GAT CAT GTT GGG TTT TAC G-3’. Amplified DNA was transferred from an agarose gel to a nylon membrane. The membrane was prehybridized for 4 hr at 65 °C in a 1X hybridized buffer (Life Technologies, Gaithersburg, MD). The 32P labeled oligonucleotide probe was added to the hybridization buffer at a final concentration of 25 nM, and hybridization was carried out for 14 hr at 5 °C below the melting temperature of the probe. Following hybridization, the membrane was washed once for 5 min in 2X SSC-0.1% (wt/vol) sodium dodecyl sulfate (SDS), twice for 30 min in 0.1X SSC-0.1% SDS at room temperature, and one for 15 min in 0.1X SSC-0.1% SDS at the melting temperature of the probe. It was exposed to X-ray film at - 70 º C for 14 hr, and the film was developed.
Results
Conjunctival lesions showed typical morphology of MALT lymphoma
All 5 cases illustrated typical morphological features of MALT lymphomas in the conjunctiva characterized by diffuse dense lymphoid infiltrates that displayed an expansive growth pattern within the marginal zone and some surrounding reactive follicles (Fig. 1). Neoplastic cells were heterogeneous, consisting of small lymphocytes, centrocyte-like cells, monocytoid cells, as well as plasma cells, and variable number of mitoses. Tumor cells infiltrated the conjunctival epithelium with formation of lymphoepithelial lesions in some areas. No intact bacteria including H. pylori and fungi were found on the sections in any of the cases studied.
Fig. 1.
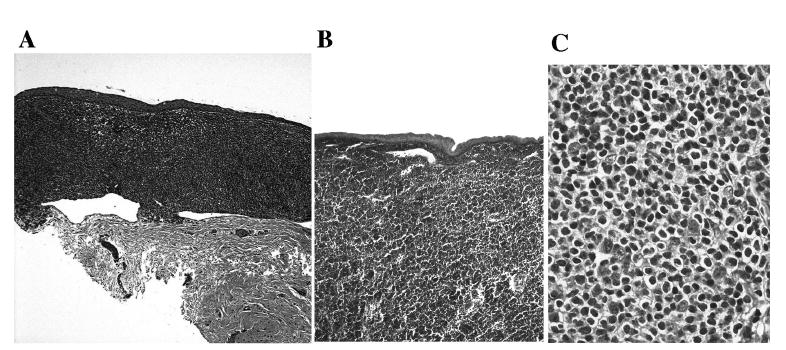
Photomicrographs showing well-demarcated MALT lymphoma infiltration in the substantia propria beneath or invading the conjunctival epithelium in Case 1 (A) and Case 3 (B); higher magnification showing cytologic features of MALT lymphoma (C) (hematoxylin & eosin, original magnification: A, × 50; B, × 100; C, × 400)
Immunohistochemistry for the available cases demonstrated that tumors were composed of mainly positive monoclonal B cells. A monotypic expression of light kappa (κ) or lambda (λ) immunoglobulin chain could be identified (Fig. 2). T cells were located mainly surrounding the germinal centers and dendritic cells were scattered throughout the lesion. On the basis of morphology and immunohistology, conjunctival MALT lymphoma of the malignant small B-cell non-Hodgkin type was diagnosed in all 5 cases.
Fig. 2.
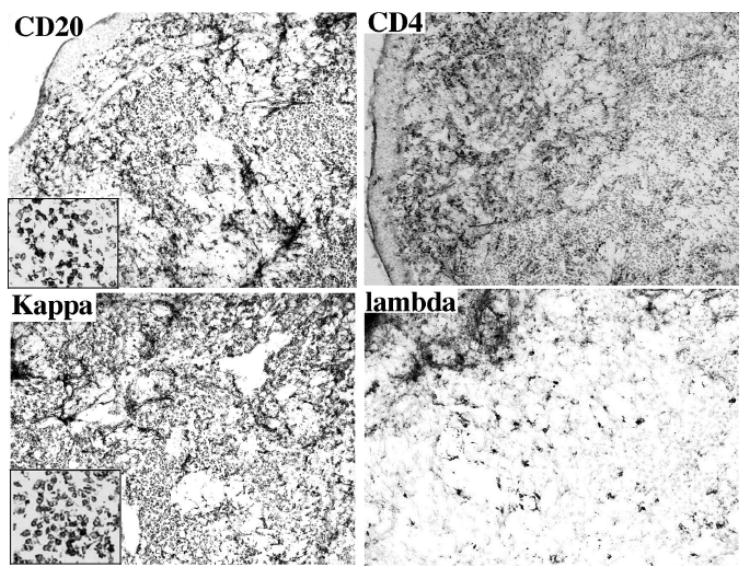
Photomicrographs showing positive CD20 and kappa staining of the conjunctival MALT lymphoma of Case 1. Note positive CD4 T-cells and lack of lambda expression in the tumor (avidin-biotin-complex immuno-peroxidase with counter-staining of methyl green, original magnification, x 200, insert, x 400
IgH gene rearrangements were demonstrated in conjunctival MALT lymphomas
The microdissected infiltrated lymphoid cells that were subjected for PCR amplification showed a same size band at the complementary determining region (CDR3) site of the IgH gene (Fig. 3). This finding confirmed the diagnosis of B-cell lymphoma (Bagg et al., 2002).
Fig. 3.

PCR amplification showing IgH gene rearrangements of CDR3 region with different primer sets in the 5 cases of conjunctival MALT lymphoma (lane 1-5, Case 1-5; lane 6, negative control; lane 7, positive control).
H. pylori gene was detected in most conjunctival MALT lymphomas
Both H. pylori urease B and vacA m2 genes were detected and confirmed using Southern hybridization in 4/5 cases (Fig. 4). The positive signal was found only from the MALT lymphoma cells. Among the 4 positive cases, one (Case 2, the 58 year-old patient) was diagnosed with H. pylori gastritis and had a positive serum antibody titer against H. pylori, one (Case 1, the 10 year-old child) had negative titer; and the other two patients (Case 4 and 5) were not tested for serum antibody. Upper endoscopy with gastric biopsy and culture of H. pylori were done only in Case 1 and the results were negative. The case without H. pylori DNA (Case 3) was the one with a history of abnormal bone marrow. In addition, H. pylori genes were not detected in non-malignant conjunctival cells from the cases of MALT lymphoma, the lymphocytes from the case with conjunctivitis, the ATL cells from the case with conjunctival HTLV-1 infection, or the lymphoma cells from the case of orbital B-cell lymphoma (Fig. 5).
Fig. 4.
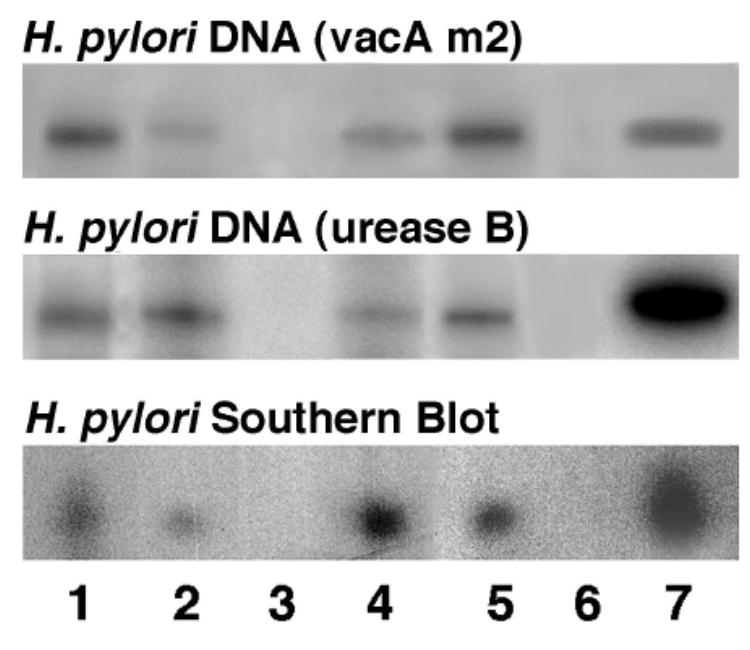
PCR and Southern blot showing detection of both H. pylori urease B and vacA m2 genes in the MALT lymphoma of Case 1-4 (lane 1-4, Case 1-4; lane 5, Case 5; lane 6, negative control; lane 7, positive control).
Fig. 5.

PCR showing absent H. pylori genes in the conjunctival resident cells, orbital MALT lymphoma, conjunctivitis, and conjunctival HTLV-1/ATL (lane 1, conjunctival MALT lymphoma cells of Case 1; lane 2, conjunctival non-lymphoma cells of Case 1; lane 3, conjunctival MALT lymphoma cells of Case 2; lane 4, inflammatory cells from conjunctivitis; lane 5, conjunctival HTLV-1/ATL cells; lane 6. orbital MALT lymphoma cells; lane 7, negative control; lane 8, positive control).
Microdissected cells from normal conjunctival epithelia and stroma (substancia propria) did not contain H. pylori genes (data not shown).
Discussion
In this study we have successfully amplified H. pylori urease B and cag/vacA m2 genes in 4/5 cases of conjunctival MALT lymphoma, but not in normal conjunctival tissues. The negative case was the one with abnormal bone marrow, thus this case may not be a de novo conjunctival MALT lymphoma. The case with negative H. pylori serology was a 10 year-old boy and this negative result may be due to the relative unreliability of serologic testing in young children ( Suerbaum and Michetti, 2002 ). The quantity of PCR products generated from the microdissected DNA template in each conjunctival lesion was less than 1 ng, below the minimum of 1-10 ng required for the visualization on an ethidium bromide-stained gel; therefore, radioisotope labeled primers and autoradiography were used. Using current technology, the minimum quantity of PCR product suitable for DNA sequencing is 10 ng (Northwoods DNA, Inc. sequencing service) to 30 ng (SeqWright DNA technology service). The PCR products from the microdissected DNA template were insufficient for sequencing and further confirmation of H. pylori infection in this study. Instead, we chose Southern blot hybridization to confirm the presence of H. pylori genes.
Interestingly, the infectious DNA was exclusively detected in the MALT lymphoma cells, not in normal conjunctival cells. In addition, the H. pylori genes were not found in normal infiltrating lymphocytes in the conjunctiva of a case with conjunctivitis. Furthermore, they were not detected in other types of ocular adnexal lymphomas, such as conjunctival ATL and orbital B-cell lymphoma. Therefore, H. pylori DNA was detected only in or around conjunctival MALT lymphoma lesions. H. pylori-associated gastric MALT lymphoma (Isaacson, 1999;Cavalli et al., 2001;Suerbaum and Michetti, 2002;Wotherspoon, 1996), is well recognized. Our findings demonstrate for the first time to our knowledge an association between H. pylori and conjunctival MALT lymphoma.
MALT lymphoma is a low-grade neoplasm of B cells that proliferate preferentially in mucosal and other extranodal sites and is characterized by a particular interaction between epithelium and reactive germinal centers (Harris, 1991). The majority of MALT lymphomas show somatic hypermutation of immunoglobulin gene variable regions, indicating that the cells have passed through the germinal center and may have undergone affinity maturation under the influence of an antigen (Qin et al., 1995). It is possible that local stimulation of lymphoma-precursor-B-cells is triggered either by an exogenous (e.g., H. pylori) or endogenous (e.g., autoantigen) antigen in cooperation with reactive inflammatory infiltration as local tumor growth is established (Parsonnet and Isaacson, 2004;Yamasaki et al., 2004). Further mutagenic alterations may then guide lymphoma development and progression.
The onset of gastric MALT lymphoma, where lymphocytes are not normally present, is preceded by the acquisition of mucosa-associated lymphoid tissue (MALT) as a result of H. pylori infection ( Zucca et al., 2000 ). The microorganism can be found in the gastric mucosa in nearly all instances of gastric MALT lymphoma, with several lines of evidence suggesting a link between H. pylori-induced chronic gastritis and the development of lymphoma. Some gastric MALT lymphomas have been shown to depend on the presence of T cells specifically activated by H. pylori antigens for continued proliferation in vitro; the importance of this stimulation in vivo has been demonstrated by the induction of remission in gastric MALT lymphomas with antibiotic treatment to eradicate H. pylori infection ( Hussell et al., 1996 ; Wotherspoon, 1996 ; Fischbach et al., 2004 ). Therefore, H. pylori may provide the antigenic stimulus for sustaining the growth of gastric lymphoma ( Cavalli et al., 2001 ). H. pylori-driven polyclonal B-cell lymphoproliferation could be an initial event of a process that could lead to a true clonal disease with time. Thus, H. pylori alone may not be directly responsible for lymphoid tumor development; additional events, encompassing genetic, immunologic, and microenvironmental factors are probably necessary in order to induce malignant B-cell formation. In other words, H. pylori may be associated with lymphomagenesis through a T cell dependent pathway that responds to a specific antigen of H. pylori.
Although no H. pylori bacteria but only its DNA was identified, the immunogenic residua include intracellular, cell wall, and DNA components. Immune response can be triggered by the DNA components by virtue of the presence of microbial unmethylated CpG motifs ( Sun et al., 2000 ). Such DNA components can activate antigen-presenting cells and stimulate immune cells to produce inflammatory cytokines ( Mutwiri et al., 2003 ). Therefore, it is possible that H. pylori DNA, by its immunogenic potential, may perpetuate chronic conjunctivitis. Further investigation of the antigenic role of H. pylori DNA is required.
H. pylori is one of the most diverse bacterial species and is naturally competent for genetic transformation ( Suerbaum et al., 1998 ; Suerbaum, 2000 ). The process of natural transformation in H. pylori is reported to be mediated by the basic components of a type IV secretion system ( Israel et al., 2000 ; Hofreuter et al., 2001 ). The type IV transporter encoded by the cag-PAI is involved in translocation of the bacterial protein CagA into human cells. H. pylori strains that process the Cag pathogenicity island induce more severe gastritis, which augments the risk for developing peptic ulcer and malignancy. H. pylori species with the CagA gene are able to induce activation of a protein kinase cascade, resulting in an increased expression of some protooncogenes, such as c-fos and c-jun in gastric epithelial cells ( Meyer-ter-Vehn et al., 2000 ). In addition, H. pylori strains harboring the cagA and vacA m2 genotype are associated with the highest risk of developing MALT lymphoma ( Koehler et al., 2003 ). The vacA m2 allele is the predominant subtype in gastric MALT lymphoma. Our data demonstrate the presence of H. pylori genes including the vacA m2, in or among the conjunctival MALT lymphoma cells. This finding suggests that the conjunctiva, in addition to the stomach, could be a mucosal site in which H. pylori may serve as a putative antigen capable of triggering MALT lymphomatous proliferation. Further experiments to demonstrate the importance of activated T cells in inducing tumor cell proliferation are required.
Recently a lower incidence (18.5%) of t(11;18)(q21;q21) translocation has been reported in 27 cases of conjunctival MALT lymphoma compared to a rate (23.9%) in 173 cases of gastric MALT lymphoma (Ye et al., 2003). CagA-positive strains of H. pylori, which are more virulent and pathogenic, were significantly more prevalent (93.3%) in t(11;18)(q21;q21)-positive gastric MALT lymphoma than in the translocation negative cases (51.9%). The translocation is influenced by the nature of pre-malignant diseases associated with MALT lymphoma. Thus, it is possible that eradication of H. pylori may be even more effective in regression of conjunctival MALT lymphoma.
In summary, H. pylori may be associated with conjunctival MALT lymphoma. Its role in lymphomagenesis needs to be further defined and the development of MALT lymphoma may require additional environmental, microbial, or host genetic factors. Helicobacter infection may play a pathologic role in the development of conjunctival MALT lymphoma. H. pylori eradication therapy may be helpful in the prevention or treatment of conjunctival MALT lymphoma.
References
- Bagg A, Braziel RM, Arber DA, Bijwaard KE, Chu AY. Immunoglobulin heavy chain gene analysis in lymphomas: a multi-center study demonstrating the heterogeneity of performance of polymerase chain reaction assays. J Mol Diagn. 2002;4:81–89. doi: 10.1016/S1525-1578(10)60685-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayerdorffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, Stolte M. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group Lancet. 1995;345:1591–1594. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- Bayerdorffer E, Miehlke S, Neubauer A, Stolte M. Gastric MALT-lymphoma and Helicobacter pylori infection. Aliment Pharmacol Ther. 1997;1(11 Suppl):89–94. doi: 10.1046/j.1365-2036.11.s1.12.x. [DOI] [PubMed] [Google Scholar]
- Cavalli F, Isaacson PG, Gascoyne RD, Zucca E. MALT Lymphomas. Hematology (Am Soc Hematol Educ Program) . 2001:241–258. doi: 10.1182/asheducation-2001.1.241. [DOI] [PubMed] [Google Scholar]
- Clayton CL, Kleanthous H, Coates PJ, Morgan DD, Tabaqchali S. Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol. 1992;30:192–200. doi: 10.1128/jcm.30.1.192-200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland SE, Foss HD, Hidayat AA, Cockerham GC, Hummel M, Stein H. Extranodal marginal zone B cell lymphomas of the uvea: an analysis of 13 cases. J Pathol. 2002;197:333–340. doi: 10.1002/path.1130. [DOI] [PubMed] [Google Scholar]
- Fischbach W, Goebeler-Kolve ME, Dragosics B, Greiner A, Stolte M. Long term outcome of patients with gastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT) following exclusive Helicobacter pylori eradication therapy: experience from a large prospective series. Gut. 2004;53:34–37. doi: 10.1136/gut.53.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbarrini A, Franceschi F. Autoimmune diseases and Helicobacter pylori infection. Biomed Pharmacother. 1999;53:223–226. doi: 10.1016/S0753-3322(99)80092-4. [DOI] [PubMed] [Google Scholar]
- Harris NL. Extranodal lymphoid infiltrates and mucosa-associated lymphoid tissue (MALT). A unifying concept. Am J Surg Pathol. 1991;15:879–884. doi: 10.1097/00000478-199109000-00008. [DOI] [PubMed] [Google Scholar]
- Harris NL, Isaacson PG. What are the criteria for distinguishing MALT from non-MALT lymphoma at extranodal sites? Am J Clin Pathol. 1999;111:S126–132. [PubMed] [Google Scholar]
- Hofreuter D, Odenbreit S, Haas R. Natural transformation competence in Helicobacter pylori is mediated by the basic components of a type IV secretion system. Mol Microbiol. 2001;41:379–391. doi: 10.1046/j.1365-2958.2001.02502.x. [DOI] [PubMed] [Google Scholar]
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996;178:122–127. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Isaacson PG. Gastric MALT lymphoma: from concept to cure. Ann Oncol. 1999;10:637–645. doi: 10.1023/a:1008396618983. [DOI] [PubMed] [Google Scholar]
- Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410–1416. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Isaacson PG, Muller-Hermelink HK, Piris MA. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World health organization classification of tumors Pathology and genetics: Tumors of haemopoietic and lymphoid tissues. ARC Press; Lyon: 2001. pp. 157–160. [Google Scholar]
- Israel DA, Lou AS, Blaser MJ. Characteristics of Helicobacter pylori natural transformation. FEMS Microbiol Lett. 2000;186:275–280. doi: 10.1111/j.1574-6968.2000.tb09117.x. [DOI] [PubMed] [Google Scholar]
- Koehler CI, Mues MB, Dienes HP, Kriegsmann J, Schirmacher P, OdenthaL M. Helicobacter pylori genotyping in gastric adenocarcinoma and MALT lymphoma by multiplex PCR analyses of paraffin wax embedded tissues. Mol Pathol. 2003;56:36–42. doi: 10.1136/mp.56.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ye H, Ruskone-Fourmestraux A, De Jong D, Pileri S, Thiede C, Lavergne A, Boot H, Caletti G, Wundisch T. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology. 2002;122:1286–1294. doi: 10.1053/gast.2002.33047. [DOI] [PubMed] [Google Scholar]
- Marshall B. Helicobacter pylori: 20 years on. Clin Med. 2002;2:147–152. doi: 10.7861/clinmedicine.2-2-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-ter-Vehn T, Covacci A, Kist M, Pahl HL. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J Biol Chem. 2000;275:16064–16072. doi: 10.1074/jbc.M000959200. [DOI] [PubMed] [Google Scholar]
- Mutwiri G, Pontarollo R, Babiuk S, Griebel P, van Drunen Littel-van den Hurk S, Mena A, Tsang C, Alcon V, Nichani A, Ioannou X. Biological activity of immunostimulatory CpG DNA motifs in domestic animals. Vet Immunol Immunopathol. 2003;91:89–103. doi: 10.1016/s0165-2427(02)00246-5. [DOI] [PubMed] [Google Scholar]
- Neubauer A, Thiede C, Morgner A, Alpen B, Ritter M, Neubauer B, Wundisch T, Ehninger G, Stolte M, Bayerdorffer E. Cure of Helicobacter pylori infection and duration of remission of low-grade gastric mucosa-associated lymphoid tissue lymphoma. J Natl Cancer Inst. 1997;89:1350–1355. doi: 10.1093/jnci/89.18.1350. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Miyajima S, Okada N. Salivary gland MALT lymphoma associated with Helicobacter pylori infection in a patient with Sjogren’s Syndrome. J Dermatol. 2000;27:450–452. doi: 10.1111/j.1346-8138.2000.tb02204.x. [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Isaacson PG. Bacterial infection and MALT lymphoma. N Engl J Med. 2004;350:213–215. doi: 10.1056/NEJMp038200. [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- Qin Y, Greiner A, Trunk MJ, Schmausser B, Ott MM, Muller-Hermelink HK. Somatic hypermutation in low-grade mucosa-associated lymphoid tissue-type B-cell lymphoma. Blood. 1995;86:3528–3534. [PubMed] [Google Scholar]
- Roggero E, Zucca E, Mainetti C, Bertoni F, Valsangiacomo C, Pedrinis E, Borisch B, Piffaretti JC, Cavalli F, Isaacson PG. Eradication of Borrelia burgdorferi infection in primary marginal zone B-cell lymphoma of the skin. Hum Pathol. 2000;31:263–268. doi: 10.1016/s0046-8177(00)80233-6. [DOI] [PubMed] [Google Scholar]
- Sharara N, Holden JT, Wojno TH, Feinberg AS, Grossniklaus HE. Ocular adnexal lymphoid proliferations: clinical, histologic, flow cytometric, and molecular analysis of forty-three cases. Ophthalmology. 2003;110:1245–1254. doi: 10.1016/S0161-6420(03)00330-0. [DOI] [PubMed] [Google Scholar]
- Shen DF, Zhuang Z, LeHoang P, Boni R, Zheng S, Nussenblatt RB, Chan CC. Utility of microdissection and polymerase chain reaction for the detection of immunoglobulin gene rearrangement and translocation in primary intraocular lymphoma. Ophthalmology. 1998;105:1664–1669. doi: 10.1016/S0161-6420(98)99036-4. [DOI] [PubMed] [Google Scholar]
- Shen DF, Herbort CP, Tuaillon N, Buggage RR, Egwuagu CE, Chan CC. Detection of Toxoplasma gondii DNA in primary intraocular B-cell lymphoma. Mod Pathol. 2001;14:995–999. doi: 10.1038/modpathol.3880424. [DOI] [PubMed] [Google Scholar]
- Suerbaum S. Genetic variability within Helicobacter pylori. Int J Med Microbiol. 2000;290:175–181. doi: 10.1016/S1438-4221(00)80087-9. [DOI] [PubMed] [Google Scholar]
- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- Suerbaum S, Smith JM, Bapumia K, Morelli G, Smith NH, Kunstmann E, Dyrek I, Achtman M. Free recombination within Helicobacter pylori. Proc Natl Acad Sci USA. 1998;95:12619–12624. doi: 10.1073/pnas.95.21.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Zhang X, Tough D, Sprent J. Multiple effects of immunostimulatory DNA on T cells and the role of type I interferons. Springer Semin Immunopathol. 2000;22:77–84. doi: 10.1007/s002810000028. [DOI] [PubMed] [Google Scholar]
- Thieblemont C, Berger F, Coiffier B. Mucosa-associated lymphoid tissue lymphomas. Curr Opin Oncol. 1995;7:415–420. [PubMed] [Google Scholar]
- Thieblemont C, Berger F, Dumontet C, Moullet I, Bouafia F, Felman P, Salles G, Coiffier B. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood. 2000;95:802–806. [PubMed] [Google Scholar]
- Wotherspoon AC. Gastric MALT lymphoma and Helicobacter pylori. Yale J Biol Med. 1996;69:61–68. [PMC free article] [PubMed] [Google Scholar]
- Wotherspoon AC, Dogan A, Du MQ. Mucosa-associated lymphoid tissue lymphoma. Curr Opin Hematol. 2002;9:50–55. doi: 10.1097/00062752-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Yamasaki R, Yokota K, Okada H, Hayashi S, Mizuno M, Yoshino T, Hirai Y, Saitou D, Akagi T, Oguma K. Immune response in Helicobacter pylori-induced low-grade gastric-mucosa-associated lymphoid tissue (MALT) lymphoma. J Med Microbiol. 2004;53:21–29. doi: 10.1099/jmm.0.05348-0. [DOI] [PubMed] [Google Scholar]
- Ye H, Liu H, Attygalle A, Wotherspoon AC, Nicholson AG, Charlotte F, Leblond V, Speight P, Goodlad J, Lavergne-Slove A. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood. 2003;102:1012–1018. doi: 10.1182/blood-2002-11-3502. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Magagnoli M, Galieni P, Martelli M, Poletti V, Zaja F, Molica S, Zaccaria A, Cantonetti AM, Gentilini P. Nongastrointestinal low-grade mucosa-associated lymphoid tissue lymphoma: analysis of 75 patients. J Clin Oncol. 1999;17:1254. doi: 10.1200/JCO.1999.17.4.1254. [DOI] [PubMed] [Google Scholar]
- Zucca E, Bertoni F, Roggero E, Cavalli F. The gastric marginal zone B-cell lymphoma of MALT type. Blood. 2000;96:410–419. [PubMed] [Google Scholar]


