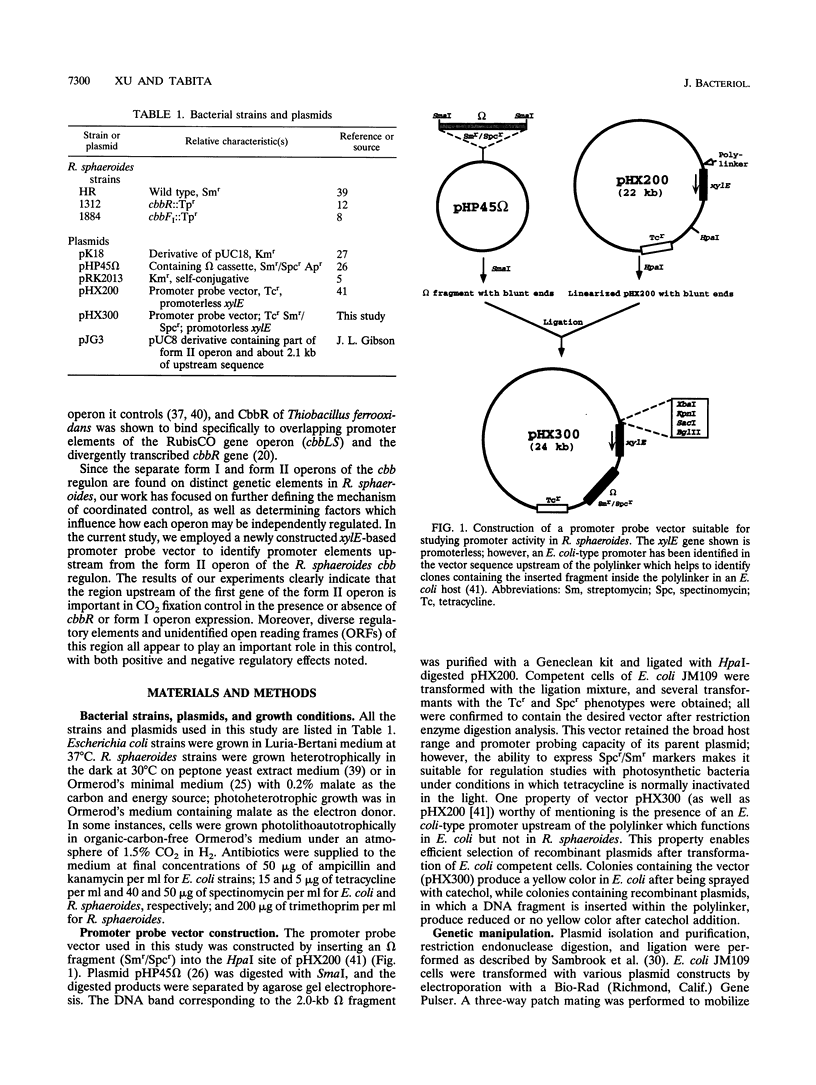
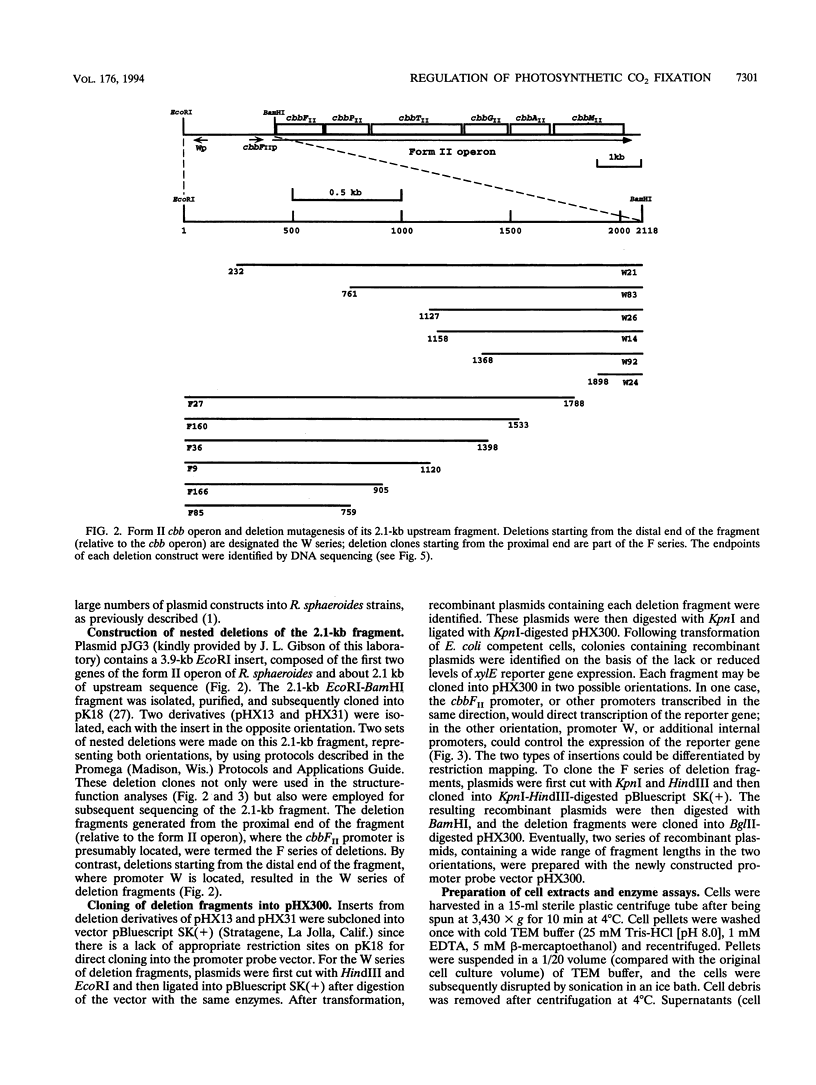
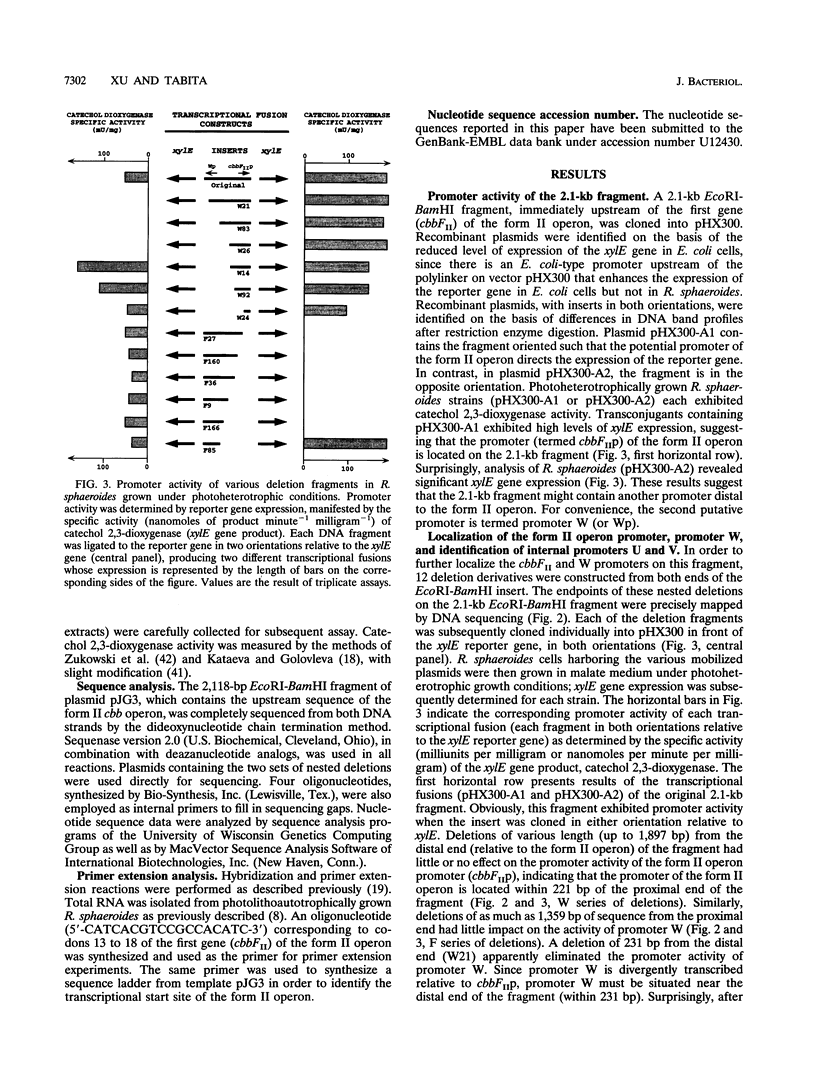
Abstract
The unlinked form I and form II Calvin cycle CO2 fixation (cbb) operons of the photosynthetic bacterium Rhodobacter sphaeroides are located on different genetic elements, yet both operons are positively regulated by the transcription activator protein CbbR, the product of the cbbR gene located immediately upstream of the form I operon. By employing deletion mutagenesis, and a newly constructed promoter probe vector, the form II operon promoter (cbbFIIp) and three other promoters (Up, Vp, and Wp) were localized within 2.1 kb upstream of the form II operon. Mutations in both cbbR and the first gene of the form I operon (cbbFI) elicited both positive and negative responses when transcriptional fusions controlled by these four promoters were examined. With the exception of Wp, all these upstream promoters were repressed by oxygen. In addition, these promoters were associated with open reading frames of unknown function whose deduced amino acid sequences showed no significant relationship to proteins in current databases. The results of these experiments suggest that the promoter sequences and genes upstream of the form II cbb operon may be intimately involved with control of the cbb regulon of this photosynthetic organism.
Full text
PDF
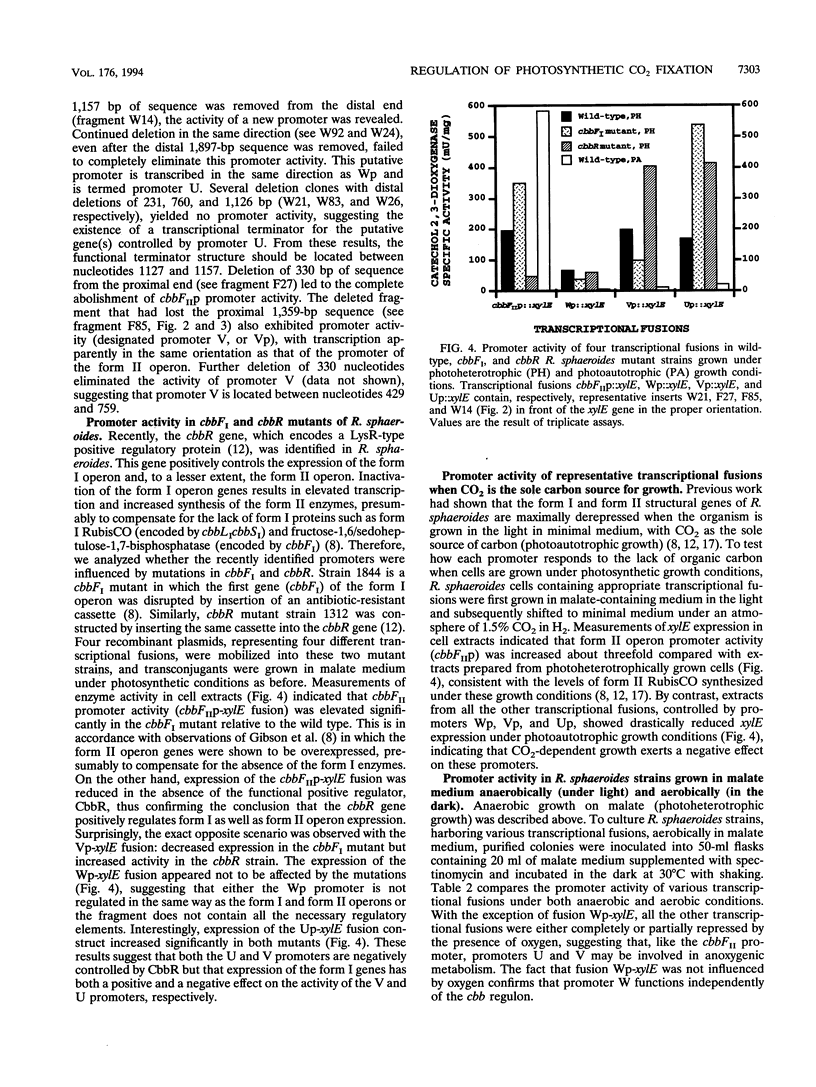
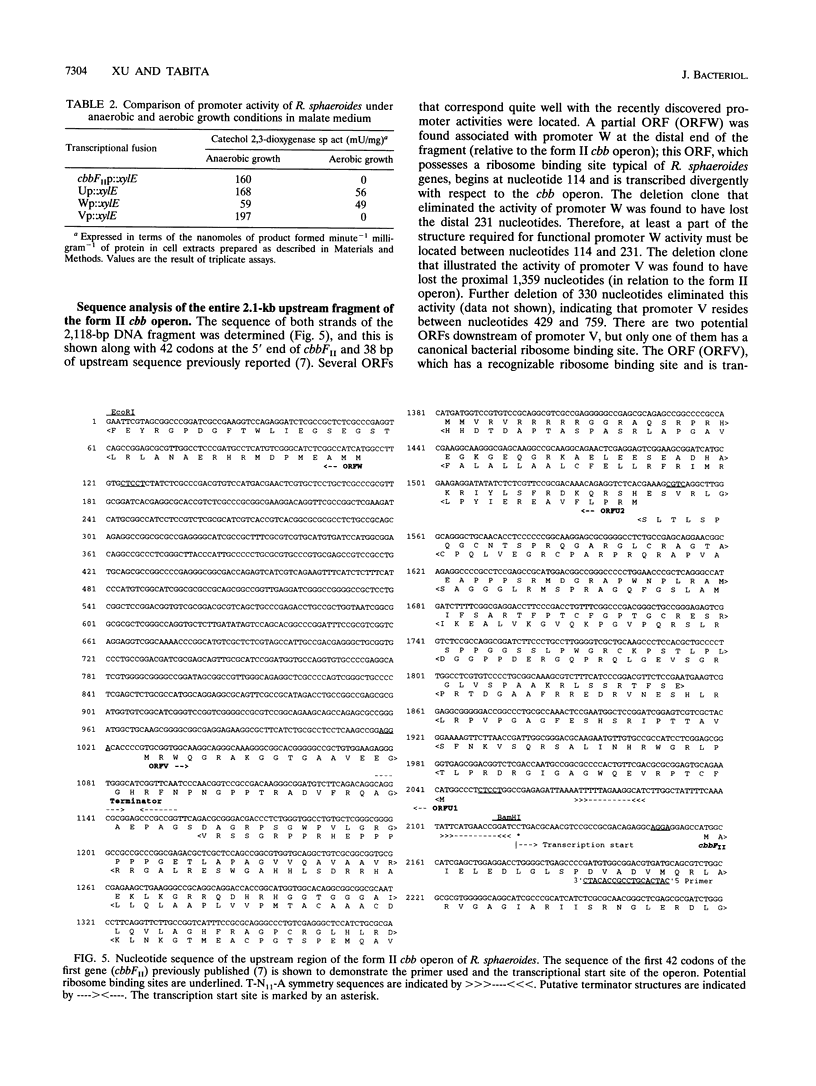
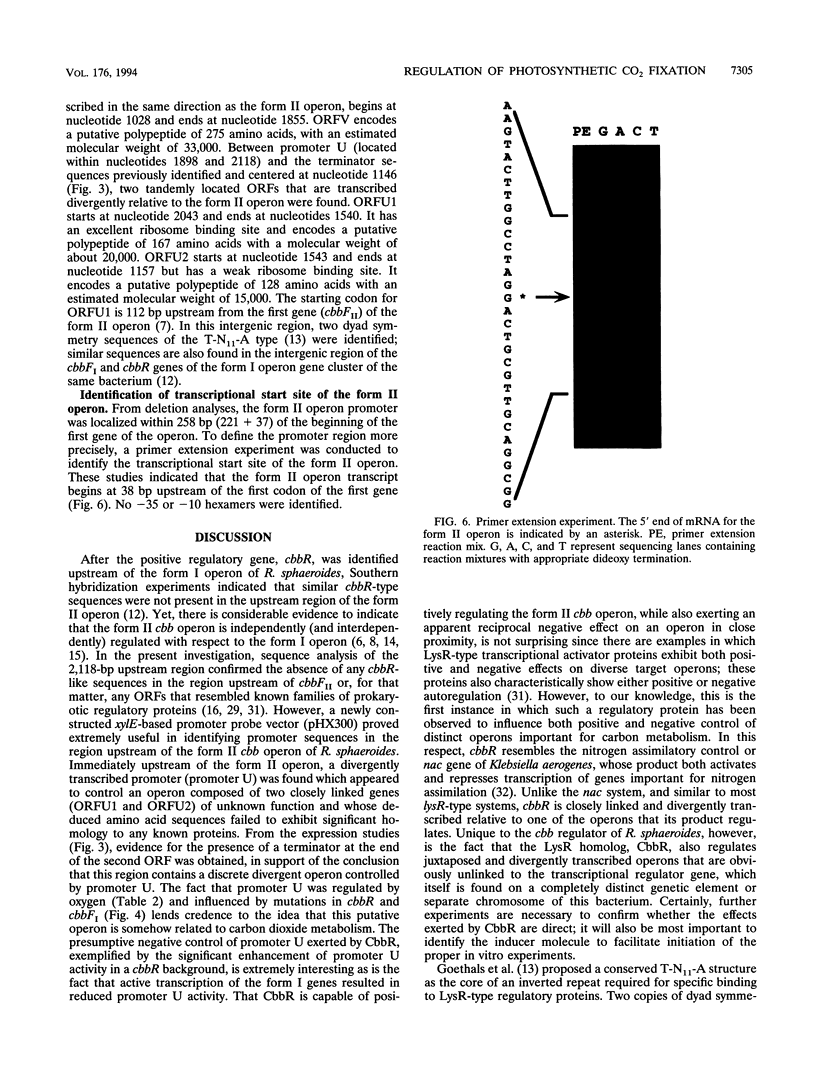

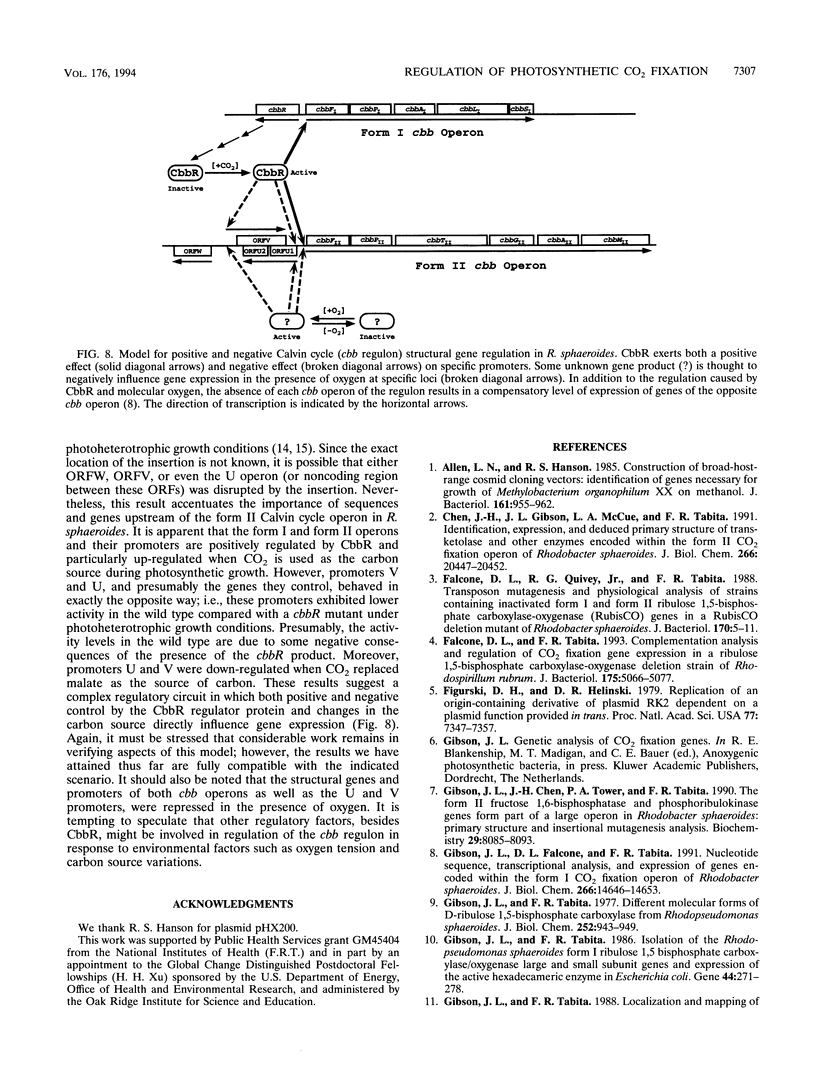

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. N., Hanson R. S. Construction of broad-host-range cosmid cloning vectors: identification of genes necessary for growth of Methylobacterium organophilum on methanol. J Bacteriol. 1985 Mar;161(3):955–962. doi: 10.1128/jb.161.3.955-962.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Gibson J. L., McCue L. A., Tabita F. R. Identification, expression, and deduced primary structure of transketolase and other enzymes encoded within the form II CO2 fixation operon of Rhodobacter sphaeroides. J Biol Chem. 1991 Oct 25;266(30):20447–20452. [PubMed] [Google Scholar]
- Falcone D. L., Quivey R. G., Jr, Tabita F. R. Transposon mutagenesis and physiological analysis of strains containing inactivated form I and form II ribulose bisphosphate carboxylase/oxygenase genes in Rhodobacter sphaeroides. J Bacteriol. 1988 Jan;170(1):5–11. doi: 10.1128/jb.170.1.5-11.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D. L., Tabita F. R. Complementation analysis and regulation of CO2 fixation gene expression in a ribulose 1,5-bisphosphate carboxylase-oxygenase deletion strain of Rhodospirillum rubrum. J Bacteriol. 1993 Aug;175(16):5066–5077. doi: 10.1128/jb.175.16.5066-5077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. L., Chen J. H., Tower P. A., Tabita F. R. The form II fructose 1,6-bisphosphatase and phosphoribulokinase genes form part of a large operon in Rhodobacter sphaeroides: primary structure and insertional mutagenesis analysis. Biochemistry. 1990 Sep 4;29(35):8085–8093. doi: 10.1021/bi00487a014. [DOI] [PubMed] [Google Scholar]
- Gibson J. L., Falcone D. L., Tabita F. R. Nucleotide sequence, transcriptional analysis, and expression of genes encoded within the form I CO2 fixation operon of Rhodobacter sphaeroides. J Biol Chem. 1991 Aug 5;266(22):14646–14653. [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Different molecular forms of D-ribulose-1,5-bisphosphate carboxylase from Rhodopseudomonas sphaeroides. J Biol Chem. 1977 Feb 10;252(3):943–949. [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Isolation of the Rhodopseudomonas sphaeroides form I ribulose 1,5-bisphosphate carboxylase/oxygenase large and small subunit genes and expression of the active hexadecameric enzyme in Escherichia coli. Gene. 1986;44(2-3):271–278. doi: 10.1016/0378-1119(86)90191-5. [DOI] [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Localization and mapping of CO2 fixation genes within two gene clusters in Rhodobacter sphaeroides. J Bacteriol. 1988 May;170(5):2153–2158. doi: 10.1128/jb.170.5.2153-2158.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Nucleotide sequence and functional analysis of cbbR, a positive regulator of the Calvin cycle operons of Rhodobacter sphaeroides. J Bacteriol. 1993 Sep;175(18):5778–5784. doi: 10.1128/jb.175.18.5778-5784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goethals K., Van Montagu M., Holsters M. Conserved motifs in a divergent nod box of Azorhizobium caulinodans ORS571 reveal a common structure in promoters regulated by LysR-type proteins. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1646–1650. doi: 10.1073/pnas.89.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. L., Lerchen R., Hessler P., Kaplan S. Phosphoribulokinase activity and regulation of CO2 fixation critical for photosynthetic growth of Rhodobacter sphaeroides. J Bacteriol. 1990 Apr;172(4):1749–1761. doi: 10.1128/jb.172.4.1749-1761.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. L., Lerchen R., Hessler P., Kaplan S. Roles of CfxA, CfxB, and external electron acceptors in regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase expression in Rhodobacter sphaeroides. J Bacteriol. 1990 Apr;172(4):1736–1748. doi: 10.1128/jb.172.4.1736-1748.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Haughn G. W., Calvo J. M., Wallace J. C. A large family of bacterial activator proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanneau Y., Tabita F. R. Independent regulation of synthesis of form I and form II ribulose bisphosphate carboxylase-oxygenase in Rhodopseudomonas sphaeroides. J Bacteriol. 1986 Feb;165(2):620–624. doi: 10.1128/jb.165.2.620-624.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataeva I. A., Golovleva L. A. Catechol 2,3-dioxygenases from Pseudomonas aeruginosa 2x. Methods Enzymol. 1990;188:115–121. doi: 10.1016/0076-6879(90)88021-2. [DOI] [PubMed] [Google Scholar]
- Kusano T., Sugawara K. Specific binding of Thiobacillus ferrooxidans RbcR to the intergenic sequence between the rbc operon and the rbcR gene. J Bacteriol. 1993 Feb;175(4):1019–1025. doi: 10.1128/jb.175.4.1019-1025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leustek T., Hartwig R., Weissbach H., Brot N. Regulation of ribulose bisphosphate carboxylase expression in Rhodospirillum rubrum: characteristics of mRNA synthesized in vivo and in vitro. J Bacteriol. 1988 Sep;170(9):4065–4071. doi: 10.1128/jb.170.9.4065-4071.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer W. G., Arnberg A. C., Enequist H. G., Terpstra P., Lidstrom M. E., Dijkhuizen L. Identification and organization of carbon dioxide fixation genes in Xanthobacter flavus H4-14. Mol Gen Genet. 1991 Feb;225(2):320–330. doi: 10.1007/BF00269865. [DOI] [PubMed] [Google Scholar]
- Muller E. D., Chory J., Kaplan S. Cloning and characterization of the gene product of the form II ribulose-1,5-bisphosphate carboxylase gene of Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jan;161(1):469–472. doi: 10.1128/jb.161.1.469-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Pridmore R. D. New and versatile cloning vectors with kanamycin-resistance marker. Gene. 1987;56(2-3):309–312. doi: 10.1016/0378-1119(87)90149-1. [DOI] [PubMed] [Google Scholar]
- Quivey R. G., Jr, Tabita F. R. Cloning and expression in Escherichia coli of the form II ribulose 1,5-bisphosphate carboxylase/oxygenase gene from Rhodopseudomonas sphaeroides. Gene. 1984 Nov;31(1-3):91–101. doi: 10.1016/0378-1119(84)90198-7. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Schell M. A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- Schwacha A., Bender R. A. The product of the Klebsiella aerogenes nac (nitrogen assimilation control) gene is sufficient for activation of the hut operons and repression of the gdh operon. J Bacteriol. 1993 Apr;175(7):2116–2124. doi: 10.1128/jb.175.7.2116-2124.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Chromosome transfer in Rhodobacter sphaeroides: Hfr formation and genetic evidence for two unique circular chromosomes. J Bacteriol. 1992 Feb;174(4):1135–1145. doi: 10.1128/jb.174.4.1135-1145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: genome size, fragment identification, and gene localization. J Bacteriol. 1989 Nov;171(11):5840–5849. doi: 10.1128/jb.171.11.5840-5849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita F. R. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol Rev. 1988 Jun;52(2):155–189. doi: 10.1128/mr.52.2.155-189.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale A. M., Kobayashi H., Akazawa T., Henikoff S. rbcR [correction of rcbR], a gene coding for a member of the LysR family of transcriptional regulators, is located upstream of the expressed set of ribulose 1,5-bisphosphate carboxylase/oxygenase genes in the photosynthetic bacterium Chromatium vinosum. J Bacteriol. 1991 Aug;173(16):5224–5229. doi: 10.1128/jb.173.16.5224-5229.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K. E., Tabita F. R. Isolation and partial characterization of Rhodopseudomonas sphaeroides mutants defective in the regulation of ribulose bisphosphate carboxylase/oxygenase. J Bacteriol. 1983 Nov;156(2):507–515. doi: 10.1128/jb.156.2.507-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windhövel U., Bowien B. Identification of cfxR, an activator gene of autotrophic CO2 fixation in Alcaligenes eutrophus. Mol Microbiol. 1991 Nov;5(11):2695–2705. doi: 10.1111/j.1365-2958.1991.tb01978.x. [DOI] [PubMed] [Google Scholar]
- Xu H. H., Viebahn M., Hanson R. S. Identification of methanol-regulated promoter sequences from the facultative methylotrophic bacterium Methylobacterium organophilum XX. J Gen Microbiol. 1993 Apr;139(4):743–752. doi: 10.1099/00221287-139-4-743. [DOI] [PubMed] [Google Scholar]
- Zukowski M. M., Gaffney D. F., Speck D., Kauffmann M., Findeli A., Wisecup A., Lecocq J. P. Chromogenic identification of genetic regulatory signals in Bacillus subtilis based on expression of a cloned Pseudomonas gene. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1101–1105. doi: 10.1073/pnas.80.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bergh E. R., Dijkhuizen L., Meijer W. G. CbbR, a LysR-type transcriptional activator, is required for expression of the autotrophic CO2 fixation enzymes of Xanthobacter flavus. J Bacteriol. 1993 Oct;175(19):6097–6104. doi: 10.1128/jb.175.19.6097-6104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]