Abstract
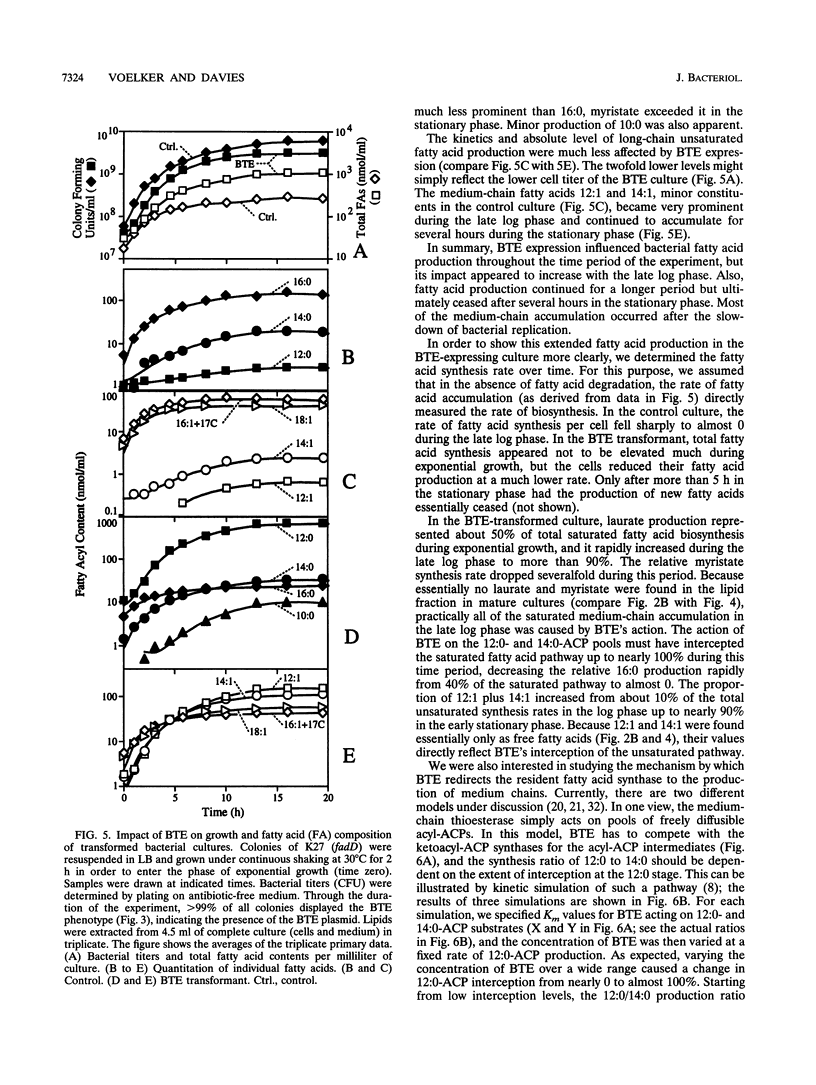
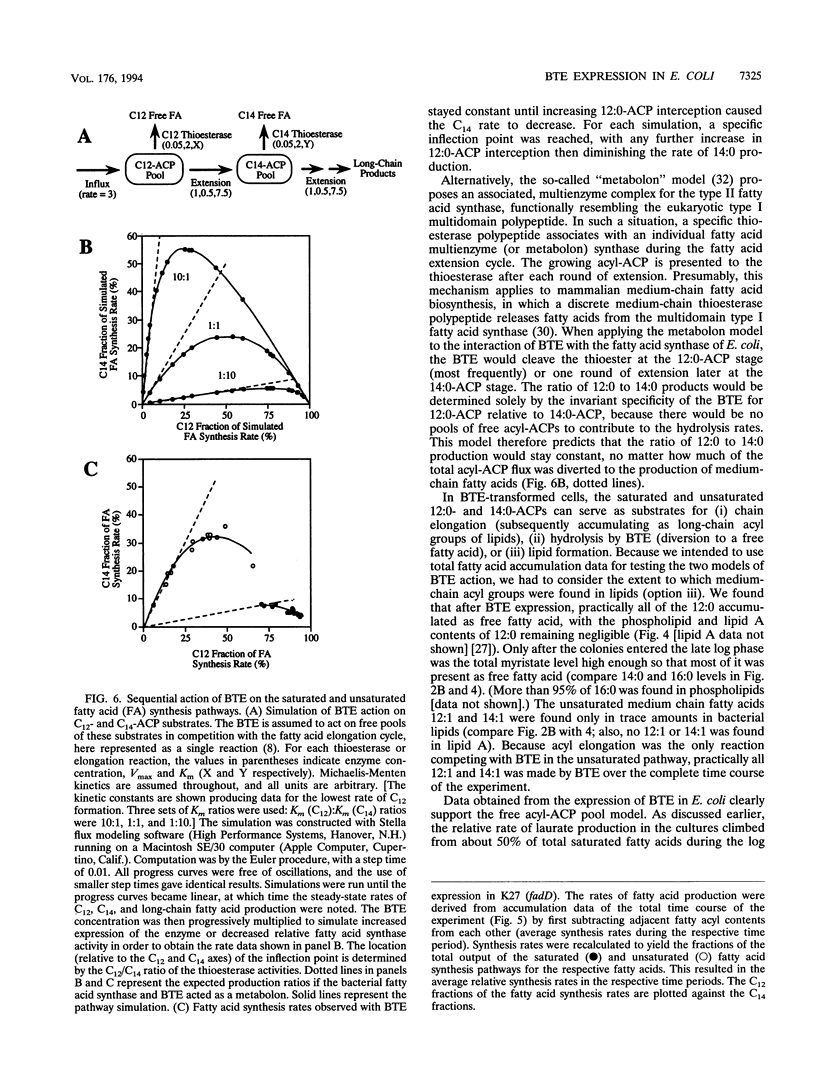
The expression of a plant (Umbellularia californica) medium-chain acyl-acyl carrier protein (ACP) thioesterase (BTE) cDNA in Escherichia coli results in a very high level of extractable medium-chain-specific hydrolytic activity but causes only a minor accumulation of medium-chain fatty acids. BTE's full impact on the bacterial fatty acid synthase is apparent only after expression in a strain deficient in fatty acid degradation, in which BTE increases the total fatty acid output of the bacterial cultures fourfold. Laurate (12:0), normally a minor fatty acid component of E. coli, becomes predominant, is secreted into the medium, and can accumulate to a level comparable to the total dry weight of the bacteria. Also, large quantities of 12:1, 14:0, and 14:1 are made. At the end of exponential growth, the pathway of saturated fatty acids is almost 100% diverted by BTE to the production of free medium-chain fatty acids, starving the cells for saturated acyl-ACP substrates for lipid biosynthesis. This results in drastic changes in membrane lipid composition from predominantly 16:0 to 18:1. The continued hydrolysis of medium-chain ACPs by the BTE causes the bacterial fatty acid synthase to produce fatty acids even when membrane production has ceased in stationary phase, which shows that the fatty acid synthesis rate can be uncoupled from phospholipid biosynthesis and suggests that acyl-ACP intermediates might normally act as feedback inhibitors for fatty acid synthase. As the fatty acid synthesis is increasingly diverted to medium chains with the onset of stationary phase, the rate of C12 production increases relative to C14 production. This observation is consistent with activity of the BTE on free acyl-ACP pools, as opposed to its interaction with fatty acid synthase-bound substrates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Black P. N., DiRusso C. C. Molecular and biochemical analyses of fatty acid transport, metabolism, and gene regulation in Escherichia coli. Biochim Biophys Acta. 1994 Jan 3;1210(2):123–145. doi: 10.1016/0005-2760(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Browse J., McCourt P. J., Somerville C. R. Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem. 1986 Jan;152(1):141–145. doi: 10.1016/0003-2697(86)90132-6. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Phospholipid alterations during growth of Escherichia coli. J Bacteriol. 1968 Jun;95(6):2054–2061. doi: 10.1128/jb.95.6.2054-2061.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. M., Anderson L., Fan C., Hawkins D. J. Developmental induction, purification, and further characterization of 12:0-ACP thioesterase from immature cotyledons of Umbellularia californica. Arch Biochem Biophys. 1991 Oct;290(1):37–45. doi: 10.1016/0003-9861(91)90588-a. [DOI] [PubMed] [Google Scholar]
- Jiang P., Cronan J. E., Jr Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action. J Bacteriol. 1994 May;176(10):2814–2821. doi: 10.1128/jb.176.10.2814-2821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Magnuson K., Jackowski S., Rock C. O., Cronan J. E., Jr Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev. 1993 Sep;57(3):522–542. doi: 10.1128/mr.57.3.522-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggert J., Witkowski A., Wessa B., Smith S. Expression in Escherichia coli, purification and characterization of two mammalian thioesterases involved in fatty acid synthesis. Biochem J. 1991 Feb 1;273(Pt 3):787–790. doi: 10.1042/bj2730787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- Rivière M. E., Arrio B., Pansu R., Faure J. Influence of the surface potential on the purple membrane structure and activity. Arch Biochem Biophys. 1991 Jan;284(1):1–8. doi: 10.1016/0003-9861(91)90253-f. [DOI] [PubMed] [Google Scholar]
- Safford R., Moran M. T., De Silva J., Robinson S. J., Moscow S., Jarman C. D., Slabas A. R. Regulated expression of the rat medium chain hydrolase gene in transgenic rape seed. Transgenic Res. 1993 Jul;2(4):191–198. doi: 10.1007/BF01977349. [DOI] [PubMed] [Google Scholar]
- Smith S. Mechanism of chain length determination in biosynthesis of milk fatty acids. J Dairy Sci. 1980 Feb;63(2):337–352. doi: 10.3168/jds.S0022-0302(80)82935-3. [DOI] [PubMed] [Google Scholar]
- Spencer A. K., Greenspan A. D., Cronan J. E., Jr Thioesterases I and II of Escherichia coli. Hydrolysis of native acyl-acyl carrier protein thioesters. J Biol Chem. 1978 Sep 10;253(17):5922–5926. [PubMed] [Google Scholar]
- Srere P. A. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Voelker T. A., Worrell A. C., Anderson L., Bleibaum J., Fan C., Hawkins D. J., Radke S. E., Davies H. M. Fatty acid biosynthesis redirected to medium chains in transgenic oilseed plants. Science. 1992 Jul 3;257(5066):72–74. doi: 10.1126/science.1621095. [DOI] [PubMed] [Google Scholar]