Abstract
Heat shock protein 70 (designated Laternula elliptica Hsp70 (LEHsp70)) expression was investigated in an Antarctic mud clam to see whether or not the inducible heat shock response has been conserved throughout over 25 million years of adaptation to constant low environmental temperatures. LEHsp70 cDNA was cloned and sequenced from the Antarctic clam Laternula elliptica. We used degenerated primers designed in the highly conserved regions of Hsp to amplify the corresponding mRNA, and full-length cDNA was obtained by rapid amplification of cDNA ends (RACE). The full length of LEHsp70 cDNA was 2470 bp, with a 5′ untranslated region (UTR) of 92 bp, a 3′ UTR of 416 bp, and an open reading frame (ORF) of 1962 bp encoding a polypeptide of 653 amino acids with an estimated molecular mass of 71.266 kDa and an estimated isoelectric point of 5.20. LEHsp70 contained highly conserved functional motifs of the cytosolic Hsp70 family. Expression of the LEHsp70 gene was quantified by quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) of digestive gland and gill tissues. Heat shock (10°C for different time periods) caused rapid induction of LEHsp70. A significant 4.6 ± 0.14-fold increase in the LEHsp70/β-Actin mRNA ratio occurred in the gill at 12 hours, which returned to baseline after 48 hours. In contrast, the maximum expression in the digestive gland (3.6 ± 0.36) was reached at 24 hours and was still significant after 48 hours (1.89 ± 0.21). This indicates that LEHsp70 may play an important role in mediating thermal stress and tolerance in this clam.
INTRODUCTION
The Antarctic clam Laternula elliptica, which is endemic to the Antarctic, is one of the most abundant macrobenthic species in the Antarctic coastal region. It has been recognized as an important sentinel species for monitoring changes in coastal ecosystems (Ahn et al 1996; Choi et al 2007; Park et al 2007). Isolated from the rest of the world, L elliptica has evolved under a cold and thermally stable environment for many millions of years. To understand the process of evolution and adaptation of this bivalve species that has survived under extremely cold environmental conditions, it is necessary to understand its biochemical and molecular mechanisms of thermal tolerance.
In the current period of global warming, temperature likely is a major factor affecting the growth and survival of L elliptica and possibly many other Antarctic organisms. Almost all organisms react to thermal stress by increasing the expression of a set of proteins known as heat shock proteins (Hsps). The heat shock response is an evolutionarily conserved mechanism for maintaining cellular homeostasis following sublethal noxious stimuli, including oxygen radicals, toxicants, and inflammatory stress (Lindquist 1986; Lindquist and Craig 1988). Disruptionof normal cellular processes can cause rapid increased synthesis of a group of proteins belonging to the Hsp families. These proteins have been classified into several families based on their molecular mass, such as Hsp90 (85–90 kDa), Hsp70 (68–73 kDa), Hsp60, Hsp47, and low molecular mass Hsps (16–24 kDa). Functionally, the Hsp90 family is involved in steroid receptor formation and protein folding (Zhao and Houry 2005); Hsp60 mediates protein stability and folding (Fink 1999); and the Hsp70 family is necessary for protein folding, multimer dissociation and association, translocation of proteins across membranes, and regulation of the heat shock response (Hartl et al 1992; James et al 1997). Many other specific functions, including a role in immunological processes, have been characterized for particular Hsp types.
Of these proteins, the Hsp70s are among the mostly highly conserved proteins known (Boorstein et al 1994). A major inducing factor for Hsp70 upregulation is the occurrence of damaged cellular protein (Feder and Hofmann 1999), and the regulation of Hsp70 gene expression occurs mainly at the transcriptional level. It is likely that Hsp70 contributes to the protection of cellular proteins by functioning as a molecular chaperone. Although little is known about the specific contribution of Hsp70 and its chaperone activity to the development of thermal tolerance, the level of this resistance correlates to the level of Hsp70 expression in individual clonal cell lines (Smith and Yaffe 1991; Li et al 1992; Parsell and Lindquist 1993).
Hsp70 genes have been cloned from several bivalve species, including the oysters Crassostrea gigas (Gourdon et al 2000; Isabelle et al 2003), C virginica (Rathinam et al 2000), and Ostrea edulis (Boutet et al 2003); a mussel, Mytilus edulis (Luedeking and Koehler 2004); and a scallop, Chlamys farreri (Wu et al 2003). Also, recent studies in different species of mollusks have reported the relevant physiological roles of heat shock gene expression in thermal tolerance (Buckley et al 2001; Rossi and Snyder 2001; Hamdoun et al 2003; Tomanek and Sanford 2003; Piano et al 2005). Interestingly, the absence of inducible Hsp gene expression by thermal stress was reported in an Antarctic fish, Trematomus bernacchii (Hofmann et al 2000), and an Antarctic ciliate, Euplotes focardii (La Terza et al 2004), which suggest that the inability of heat shock to induce Hsp supports the loss of the regulation pathway in the Hsp gene expression during evolutionary history; therefore, the characterization of the heat shock response in Antarctic ectothermal organisms is likely to be essential for elucidating their evolution and adaptation. Here we report the full Hsp70 cDNA sequence and its deduced amino acid sequence in L elliptica. In addition, we examine whether L elliptica has at all preserved capacities for inducing a heat shock response and the relative pattern of Hsp70 mRNA expression on acute warming.
MATERIALS AND METHODS
Organisms and heat exposure experiments
Laternula elliptica (shell length ≈ 80 mm) were hand collected by scuba divers from depths of 20 to 30 m in Marian cove near King Sejong Station on the northern Antarctic Peninsula (62°13′S, 58°47′W) in January 2006. After acclimation to experimental conditions (ca. 1.0°C) for 2 days, the clams were thermally challenged at 10°C over a 48-hour period without feeding.
Hsp70 cDNA cloning
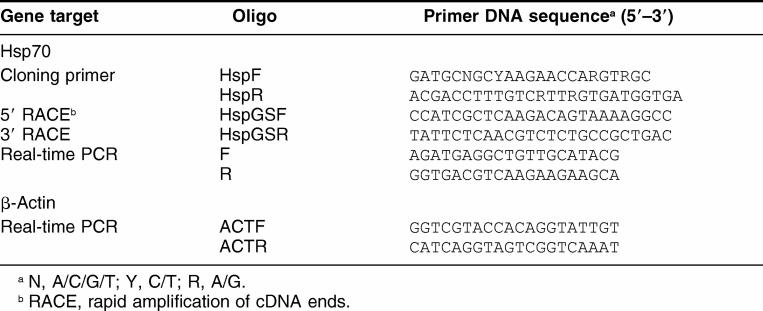
Total RNA was extracted from the digestive gland and gill using Trizol reagent (Invitrogen Co, Grand Island, NY, USA). Concentration of total RNA was determined by measuring ultraviolet (UV) absorbance at 260 nm. RNA purity was checked by determining the A260/A280 ratio, and its integrity was checked by formaldehyde agarose gel electrophoresis. Single-strand cDNA was synthesized from 1 mg total RNA in a final volume of 20 μL containing 50 pM Oligo-dT20, 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 50 mM DTT, 0.75 U Rnasin, 0.2 mM of each dNTP, and 1 U MMLV reverse transcriptase (Promega, Madison, WI, USA). Reactions were incubated for 90 minutes at 42°C and terminated by heating at 95°C for 5 minutes. Degenerated primers for amplifying an LEHsp70 cDNA fragment of about 1300 bp from L elliptica were designed on the basis of known mollusk Hsp70s (Table 1). A polymerase chain reaction (PCR) was performed with 0.1 μg of cDNA as a template in PCR buffer containing 3 mM MgCl2, 0.2 mM dNTPs, and 0.2 μM of each primer in 50 μL PCR was carried out at 94°C for 2 minutes, followed by 30 cycles of 94°C for 30 seconds, 58°C for 30 seconds, 72°C for 2 minutes, and a final extension at 72°C for 10 minutes. The PCR products were gel-purified and subcloned into pCR2.1-TOPO (Invitrogen).
Table 1.
Sequences, directions, and positions of polymerase chain reaction (PCR) primers used for the cloning of a full Hsp70 cDNA of Laternula elliptica
Rapid amplification of cDNA ends
Sequences obtained by reverse transcriptase (RT)–PCR were used to design specific oligonucleotides to perform 3′ and 5′ rapid amplification of cDNA ends ([RACE], Table 1). The RACE reactions were performed according to the instructions of the Capfishing full-length cDNA kit (Seegene, Seoul, Korea). Full-length first-strand cDNA was synthesized with oligo (dT)–annealing control primer (ACP) with the following amplification conditions: 0.2 mM each of dNTP, 10 mM Tris–HCl (pH 8.8), 50 mM KCl, 1.5 mM MgCl2, 0.2 μM of genespecific primers, 0.2 μM of 5′/3′-RACE primers, and 1 unit of Taq DNA polymerase. The program for PCR amplification was as follows: 1 cycle at 94°C for 5 minutes, 50°C for 1 minute, and 72°C for 2 minutes; 35 cycles at 94°C for 30 seconds, 58°C for 30 seconds, 72°C for 2 minutes; and a final extension at 72°C for 10 min. The resulting RACE products were separated on a 1.5% agarose gel and subcloned into pCR2.1-TOPO (Invitrogen).
Real time RT-PCR
The mRNA levels of LEHsp70 were measured by real-time quantitative RT-PCR amplification. PCR amplifications were performed in 25-μL reactions containing cDNA generated from 2.5 ng of original RNA template, 0.2 μM each of gene-specific forward and reverse primer (Table 1), and 12.5 μL of 2× QuantiTect SYBR Green PCR Mix (Takara, Tokyo, Japan). The amplified signals were detected continuously with the Smart Cycler System II (Cepheid, Sunnyvale, CA) and were detected with SYBR Green as a double-stranded DNA-specific fluorescent dye that is included in the SYBR Green qPCR premix (Takara). The amplification protocol was as follows: initial 15-second denaturation and enzyme activation at 95°C, 45 cycles of 95°C for 5 seconds, 52°C for 15 seconds, and 72°C for 15 seconds. The β-actin gene of L elliptica was used as a reference to normalize the expression levels between samples. All data were expressed relative to β-actin to compensate for any difference in reverse transcriptase efficiency. All experiments were analyzed in triplicate. Data were collected as Ct (PCR cycle number where fluorescence is detected above a threshold and decreases linearly with increasing input target quantity) using Smart Cycler optical system software version 2.0 (Cepheid, Sunnyvale, CA, USA). The Ct of each sample was used to calculate ΔCt values (target gene Ct subtracted from β-actin Ct). The relative gene expression–fold change was determined by the 2-ddCt method (Livak and Schmittgen 2001). All data were expressed as the means ± standard deviation (SD) and analyzed by an unpaired Student t-test after normalization. Differences were considered statistically significant at P < 0.05.
DNA sequence analysis
The sequences of RT–PCR and RACE products, cloned in pCR2.1-TOPO, were sequenced on a fluorescent automated sequencer from both the 5′ and 3′ ends with the ABI PRISM dye terminator cycle sequencing ready reaction kit (Applied Biosystems, Foster City, CA, USA). Complete sequences were analyzed by searching for similarities using the BLASTX search program in the National Center for Biotechnology Information (NCBI) GeneBank.
RESULTS
cDNA cloning and sequencing of the LEHsp70 gene of L elliptica
Hsp70 cDNA was amplified by degenerated primers HspF and HspR (Table 1). Primers were based on regions that are highly conserved in mollusk Hsp70. The sequence comparison of the cDNA fragment obtained confirmed the close similarity to known Hsp70 genes. The complete coding sequence of L elliptica was obtained by 3′ and 5′ RACE-PCR. The partial Hsp70 sequence was used to design new specific primers (HspGSF and HspGSR) to perform these amplifications (Table 1). The complete cDNA of the L elliptica Hsp70 gene was deposited in GenBank under accession number EF198332.
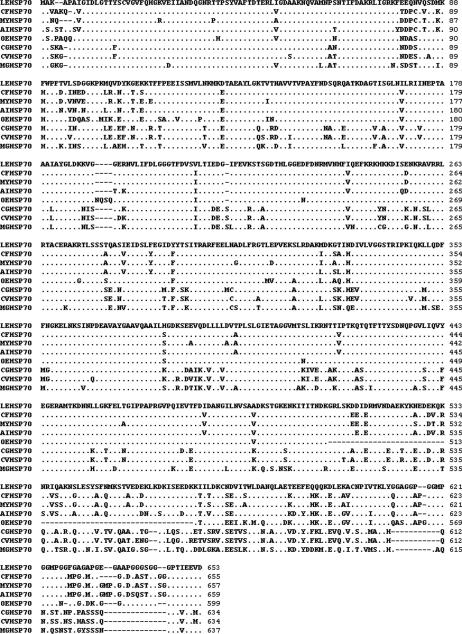
The full length of LEHsp70 cDNA from L elliptica is 2470 bp, with a single open-reading frame (ORF) of 1962 bp that encodes a protein of 653 amino acids (Fig 1). The theoretical molecular weight of LEHsp70 based on the deduced amino acid sequence was calculated to be 71.266 kDa with an isoelectronic point (pI) of 5.20. LEHsp70 cDNA includes a 5′ untranslated region (UTR) located 92 bp upstream of the putative start codon (ATG) and a 3′ UTR of 395 nucleotides that ends in a poly (A) tail. A possible consensus signal sequence for polyadenylation (AATAAA) is located 14 bp upstream of the poly(A) tail. The deduced amino acid sequence of LEHsp70 cDNA includes 3 typical motifs of the Hsp70 family at residues 9–16 (IDLGTTYS), 199–206 (DLGGGTFD), and 334–339 (IVLVGG). The putative ATP-GTP binding site motif is located at position 131–138, and the putative bipartite nuclear localization signal involved in the selective translocation of LEHsp70 into the nucleus (KK and RRLRT at positions 250–251 and 261–265, respectively). Also highly conserved, the cytoplasmic Hsp70 carboxyl terminal region of EEVD is included at position 650–653 (Fig 1).
Fig 1.
Nucleotide and deduced amino acid sequences of Laternula elliptica heat shock protein (Hsp) 70 cDNA. The consensus polyadenylation signal (AUUAAA) is double underlined. The characteristic motifs of the Hsp70 family are underlined: three signatures at positions 9–16, 199–206, and 334–339; a putative ATP-GTP binding site at 131–138; a putative bipartite nuclear localization signal at 250–251 and 261–265; and the cytoplasmic Hsp70 carboxyl terminal region at 651–654. The 3′ and 5′ untranslated regions are in lower case. Positions of the probes used in semiquantitative reverse transcriptase–polymerase chain reaction (RT–PCR) to amplify the 97-bp fragment are shown in gray
Primary structure comparison
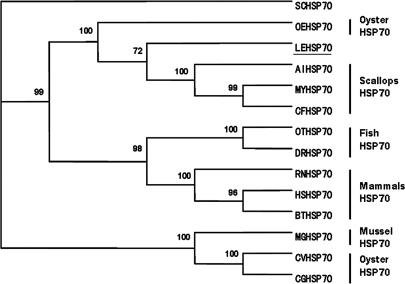
The BLAST program analysis showed that the nucleotide sequence of the LEHsp70 gene shares homology with other known Hsp70 genes, indicating that the cloned gene encodes Hsp70 protein. The predicted amino acid sequence from this cDNA revealed that it was highly conserved, and the conservation of the N-terminus was higher than that of the C-terminus (Fig 2). Compared to other invertebrate Hsp70 genes, the nucleotide sequence had 64%–76% identity, but its deduced amino acid sequence had 71%–87% identity. LEHsp70 shows greatest homology with Hsp70 from the scallop Chlamys farreri (76% cDNA identity, 87% protein identity; 94% protein similarity). Based on the sequence of LEHsp70, a phylogenetic tree was constructed using the programs CLUSTAL X1.83 and PAUP 4.0 (Fig 3). LEHsp70 clustered together with that of C farreri and an oyster, Ostrea edulis. The relationships displayed in the phylogenic tree were in general agreement with traditional taxonomy.
Fig 2.
Multiple alignments of the deduced amino acid sequences of LEHsp70 with heat shock protein (Hsp) 70s of other known bivalves. The Hsp abbreviations, species, and the GenBank accession numbers are as follows: CFHsp70, Chlamys farreri, AY206871; MYHsp70, Mizuhopecten yessoensis, AY485262; AIHsp70, Argopecten irradians, AY485261; OEHsp70, Ostrea edulis, CAC83010; CGHsp70, Crassostrea gigas, AAD31042; CVHsp70, Crassostrea virginica, CAB89802; MGHsp70, and Mytilus galloprovincialis, CAE51348
Fig 3.
Phylogenetic tree of heat shock protein (Hsp) 70 family members constructed with the neighbor-joining method. Numbers at each branch indicate the percentage of times a node was supported in 1000 bootstrap pseudoreplications by neighbor joining. The Hsp abbreviations, species, and the GenBank accession numbers are as follows: SCHsp70, Saccharomyces cerevisiae, AAA63574; RNHsp70, Rattus norvegicus, AAA17441; HSHsp70, Homo sapiens, AAA52697; BTHsp70, Bos taurus, AAA03451; DRHsp70, Danio rerio, AAH56709; OTHsp70, Oncorhynchus tshawytscha, Q91233; the other abbreviations are as in Fig 2
Expression of Hsp70 in response of thermal stress
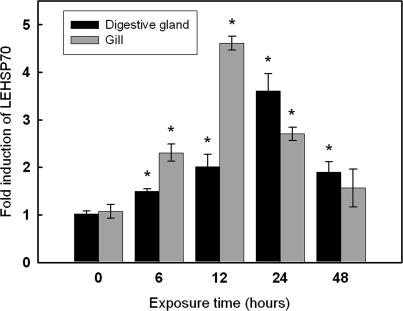
Semiquantitative RT-PCR was used to examine the time-dependent expression pattern of LEHsp70 in the digestive gland and gill of L elliptica under thermal stress. The mRNA expression of LEHsp70 at different time points after thermal treatment (10°C) are shown in Figure 4. The mRNA transcript of LEHsp70 could be detected both in the control and treatment groups. In previous work, we cloned the β-actin mRNA partial sequence from L elliptica (Park et al., unpublished data, accession number EF198331), and we used β-actin as an internal standard and normalization to verify the successful transcription and to calibrate the cDNA template for corresponding samples. After 6 hours of heat treatment, the expression of the LEHsp70 gene was upregulated and reached the highest level at 12 hours in the gill. After 24 hours, the expression of LEHsp70 decreased gradually and returned to the control level. However, the expression of the LEHsp70 gene reached its highest point at 24 hours in the digestive gland where it maintained a relatively high level.
Fig 4.
mRNA expression level of LEHsp70 at different time points after heat treatment. Transcript levels for all samples were assessed by semiquantitative reverse transcriptase–polymerase chain reaction (RT–PCR) with SYBR Green, and the relative expression of Hsp70 was obtained relative to β-actin expression. Values are expressed as means ± standard deviation of the relative variations (fold induction) between each treatment and control sample; asterisks above the bars indicate differences from the control sample that are statistically significant (*P < 0.05)
DISCUSSION
Antarctic clams are thought to have gained a unique adaptive mechanism to extremely cold environments during several million years of evolution. To address the possibility that such cold-adapted species retain a highly conserved cellular response to heat stress, we determined the full-length sequence of Hsp70 cDNA from Laternula elliptica, the first Hsp70 gene sequence reported for an Antarctic clam. The full-length cDNA of the LEHsp70 gene is 2470 bp, which encodes a polypeptide of 653 amino acids with several highly conserved functional motifs, such as 3 conserved Hsp70 family signatures, an ATP/ GTP binding motif, and a bipartite nuclear targeting sequence. In addition, a notable feature of LEHsp70 is the presence of 2 consecutive repeats of the tetrapeptide motif GGMP and cytosolic Hsp70-specific motif EEVD at the C-terminus. Structural and phylogenetic features of LEHsp70 suggest that it encodes cytosolic Hsp70. Although regions of homology extend throughout the Hsp70s, the C-terminal region has greater divergence. The variation in sequences in the C-terminus has functional consequences in proteins that may determine the functional specificity of individual Hsps. Biochemical and structural analyses showed that Hsp70 consists of a highly conserved N-terminal, 44-kDa, ATP-binding and ATPase activity domain, a less well conserved, 18-kDa, peptide-binding domain involved in the interaction with cochaperones, and a C-terminal, 10-kDa, variable domain that may form a lid over the peptide-binding pocket (Rü diger et al 1997; Zhu et al 1996). Remarkably, the C-terminal domain of LEHsp70 from residues 546 to 653 has a high glycine content (18.52%). The variations in sequence in the C-terminal region have functional consequences in proteins, as they drastically modify the position of the helical subdomain that appears to function as a latch for access to the binding site (Fuertesa et al 2004). Although the function of the C-terminal 10-kDa domain has not been revealed, this may indicate that the C-terminal domain of glycine-rich repetitions of the tetrapeptide GGMP of unknown function and the EEVD motif at the extreme C-terminus together form a structural entity that mediates cofactor binding. Unfortunately, few studies of the molecular or biochemical characterization the Hsp70 gene of Antarctic bivalves are available, and we do not know whether such a C-terminal feature is responsible for their evolutionary cold adaptation.
Several studies have confirmed the rapid expression of proteins of the Hsp70 family in aquatic organisms as a result of osmotic (Smith et al 1999), heavy metal (Geraci et al 2004), and thermal (Piano et al 2002, 2004) stress. Franzellitti and Fabbri (2005) showed that Hsp70 isoforms were induced immediately after 1 hour of heat shock in a mussel. Also, Piano et al (2002) reported that Hsp expression in response to heat shock was detectable up to 14 days in elevated protein levels. The analysis of LEHsp70 expression showed that LEHsp70 was expressed ubiquitously in both gill and digestive gland, indicating that LEHsp70 also is synthesized under unstressed conditions. LEHsp70 expression significantly increased after thermal exposure of L elliptica to 10°C and remained at a steady level or decreased after 24 h. LEHsp70 mRNA clearly was detectable in both tissues 6 hours after heat stress, whereas a lag time was needed for its expression in the digestive gland. Although it is difficult to distinguish whether the transcript can be simultaneously or differentially modulated by stress, the gill is likely the most sensitive organ in that it is directly exposed to the external environment. Thus, the organ-specific response to thermal stress found in this study is in good agreement with previous studies in other mollusks (Franzellitti and Fabbri 2005; Piano et al 2005).
Global warming is one of the major factors affecting survival, especially of Antarctic species like L elliptica that live in thermally stable environments. Hsps have received the most attention in organisms as part of a suite of stress indices because their expression is highly variable in the presence or absence of stimuli. Our study demonstrates the first complete unit of the Hsp70 gene in an Antarctic clam. Our findings suggest that the LEHsp70 gene is expressed in response to thermal stress, and therefore may play an important role in thermal tolerance. Furthermore, comparative studies between Antarctic species with and without inducible Hsp genes can provide clues to understanding adaptation and evolutionary processes in this extremely harsh environment.
Acknowledgments
We thank the diver Seung Goo Ra and the members of the 19th Korea Antarctic Research Program for their support in conducting the experiment. This study was supported by Monitoring on Environmental Changes at the Korean Arctic and Antarctic Stations (PE07040) and funded by Korea Polar Research Institute (KOPRI) in the Korea Ocean Research and Development Institute (KORDI).
REFERENCES
- Ahn I-Y, Lee SH, Kim KT, Shim JH, Kim DY. Baseline heavy metal concentrations in the Antarctic clam Laternula elliptica in Maxwell Bay, King George Island, Antarctica. Mar Pollut Bull. 1996;32:592–598.0025-326X(1996)032[0592:BHMCIT]2.0.CO;2 [Google Scholar]
- Boorstein WR, Ziegelhoffer T, Craig EA. Molecular evolution of the Hsp70 multigene family. J Mol Evol. 1994;38:1–17. doi: 10.1007/BF00175490.0022-2844(1994)038[0001:MEOTHM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Boutet I, Tanguy A, Moraga D. Organization and nucleotide sequence of the European flat oysters Ostrea edulis heat shock cognate 70 (hsc70) and heat shock protein 70 (Hsp) genes. Aquat Toxicol. 2003;65:221–225. doi: 10.1016/s0166-445x(03)00137-1.0166-445X(2003)065[0221:OANSOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Buckley BA, Owen ME, Hofmann GE. Adjusting the thermostat: the threshold induction temperature for the heat shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J Exp Biol. 2001;204:3571–3579. doi: 10.1242/jeb.204.20.3571.0022-0949(2001)204[3571:ATTTTI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Choi HJ, Ji JY, Chung KH, Ahn I-Y. Cadmium bioaccumulation and detoxification in the gill and digestive gland of the Antarctic bivalve Laternula elliptica. Comp Biochem Physio C. 2007;145:227–235. doi: 10.1016/j.cbpc.2006.12.005.1367-8280(2007)145[0227:CBADIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243.0066-4278(1999)061[0243:HSPMCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425.0031-9333(1999)079[0425:CPF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Franzellitti S, Fabbri E. Differential Hsp70 gene expression in the Mediterranean mussel exposed to various stressors. Biochem Biophys Res Commun. 2005;336:1157–1163. doi: 10.1016/j.bbrc.2005.08.244.0006-291X(2005)336[1157:DHGEIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fuertesa MA, Peŕezb JM, Sotoa M, Menéndezc M, Alonsoa C. Thermodynamic stability of the C-terminal domain of the human inducible heat shock protein 70. Biochim Biophys Acta. 2004;1699:45–56. doi: 10.1016/j.bbapap.2003.12.007.0006-3002(2004)1699[0045:TSOTCD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Geraci F, Pinsino A, Turturici G, Savona R, Giudice G, Sconzo G. Nickel, lead, and cadmium induce differential cellular responses in sea urchin embryos by activating the synthesis of different Hsp70s. Biochem Biophys Res Commun. 2004;322:873–877. doi: 10.1016/j.bbrc.2004.08.005.0006-291X(2004)322[0873:NLACID]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gourdon I, Gricourt L, Kellner K, Roch P, Escoubas JM. Characterization of a cDNA encoding a 72 kDa heat shock cognate protein (Hsc72) from the Pacific oyster, Crassostrea gigas. DNA Seq. 2000;11:265–270. doi: 10.3109/10425170009033241.1042-5179(2000)011[0265:COACEA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hamdoun AM, Cheney DP, Cherr GN. Phenotypic plasticity of Hsp70 and Hsp70 gene expression in the Pacific oyster (Crassostrea gigas): implications for thermal limits and induction of thermal tolerance. Biol Bull. 2003;205:160–169. doi: 10.2307/1543236.0006-3185(2003)205[0160:PPOHAH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartl FU, Martin J, Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453.1056-8700(1992)021[0293:PFITCT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Buckley BA, Airaksinen S, Keen JE, Somero GN. Heat shock protein expression is absent in the Antarctic Fish Trematomus bernacchii (Family Nototheniidae) J Exp Biol. 2000;203:2331–2339. doi: 10.1242/jeb.203.15.2331.0022-0949(2000)203[2331:HSPEIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Isabelle B, Arnaud T, Sabrina R, Michel A, Dario M. Molecular identification and expression of heat shock cognate 70 (Hsc70) and heat shock protein 70 (Hsp70) genes in the Pacific oyster Crassostrea gigas. Cell Stress Chaperones. 2003;8:76–85. doi: 10.1379/1466-1268(2003)8<76:miaeoh>2.0.co;2.1466-1268(2003)008[0076:MIAEOH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Pfund C, Craig EA. Functional specificity among Hsp70 molecular chaperones. Science. 1997;275:387–389. doi: 10.1126/science.275.5298.387.0193-4511(1997)275[0387:FSAHMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- La Terza A, Miceli C, Luporini P. The gene for the heat shock protein 70 of Euplotes focardii, an Antarctic psychrophilic ciliate. Antarct Sci. 2004;16:23–28.0954-1020(2004)016[0023:TGFTHS]2.0.CO;2 [Google Scholar]
- Li GC, Li L, Liu RY, Rehman M, Lee WMF. Heat shock protein Hsp70 protects cells from thermal stress even after deletion of its ATP-binding domain. Proc Natl Acad Sci USA. 1992;89:2036–2040. doi: 10.1073/pnas.89.6.2036.1091-6490(1992)089[2036:HSPHPC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443.0066-4154(1986)055[1151:THSR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215.0066-4197(1988)022[0631:THSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262.1046-2023(2001)025[0402:AORGED]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Luedeking A, Koehler A. Regulation of expression of multixenobiotic resistance (MXR) genes by environmental factors in the blue mussel Mytilus edulis. Aquat Toxicol. 2004;69:1–10. doi: 10.1016/j.aquatox.2004.03.003.0166-445X(2004)069[0001:ROEOMR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Park H, Ahn I-Y, Choi HJ, Pyo SH, Lee HE. Cloning, expression, and characterization of metallothionein from the Antarctic clam Laternula elliptica. Protein Expres Purif. 2007;52:82–88. doi: 10.1016/j.pep.2006.08.008.1046-5928(2007)052[0082:CEACOM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253.0066-4197(1993)027[0437:TFOHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Piano A, Asirelli C, Caselli F, Fabbri E. Hsp70 expression in thermally stressed Ostrea edulis, a commercially important oyster in Europe. Cell Stress Chaperones. 2002;7:250–257. doi: 10.1379/1466-1268(2002)007<0250:heitso>2.0.co;2.1466-1268(2002)007[0250:HEITSO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano A, Franzellitti S, Tinti F, Fabbri E. Sequencing and expression pattern of inducible heat shock gene products in the European flat oyster, Ostrea edulis. Gene. 2005;361:119–126. doi: 10.1016/j.gene.2005.06.034.0378-1119(2005)361[0119:SAEPOI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Piano A, Valbonesi P, Fabbri E. Expression of cytoprotective proteins, Hsp70, and metallothioneins in tissues of Ostrea edulis exposed to heat and heavy metals. Cell Stress Chaperones. 2004;9:134–142. doi: 10.1379/483.1.1466-1268(2004)009[0134:EOCPHA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam AV, Chen TT, Grossfeld RM. Cloning and sequence analysis of a cDNA for an inducible 70 kDa heat shock protein (Hsp70) of the American oyster (Crassostrea virginica) DNA Seq. 2000;11:261–264. doi: 10.3109/10425170009033240.1042-5179(2000)011[0261:CASAOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rossi S, Snyder MJ. Competition for space among sessile marine invertebrates: changes in Hsp70 expression in two pacific cnidarians. Biol Bull. 2001;201:385–393. doi: 10.2307/1543616.0006-3185(2001)201[0385:CFSASM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rüdiger S, Buchberger A, Bukau B. Interaction of Hsp70 chaperones with substrates. Nat Struct Biol. 1997;4:342–349. doi: 10.1038/nsb0597-342.1072-8368(1997)004[0342:IOHCWS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith TR, Tremblay GC, Bradley TM. Hsp70 and a 54 kDa protein (Osp54) are induced in salmon (Salmo salar) in response to hyperosmotic stress. J Exp Zool. 1999;284:286–298. doi: 10.1002/(sici)1097-010x(19990801)284:3<286::aid-jez6>3.0.co;2-j.1097-010X(1999)284[0286:HAAKPO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Smith BJ, Yaffe MP. Uncoupling thermotolerance from the induction of heat shock protein. Proc Natl Acad Sci USA. 1991;88:11091–11094. doi: 10.1073/pnas.88.24.11091.1091-6490(1991)088[11091:UTFTIO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek L, Sanford E. Heat shock protein 70 (Hsp70) as a biochemical stress indicator: an experimental field test in two congeneric intertidal gastropods (genus: Tegula) Biol Bull. 2003;205:276–284. doi: 10.2307/1543291.0006-3185(2003)205[0276:HSPHAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wu L, Song L, Xu W. Identification and cloning of heat shock protein 70 gene from scallop Chlamys farreri. High Technol Lett. 2003;13:75–79. [Google Scholar]
- Zhao R, Houry WA. Hsp90: a chaperone for protein folding and gene regulation. Biochem Cell Biol. 2005;83:703–710. doi: 10.1139/o05-158.1208-6002(2005)083[0703:HACFPF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata C, Gottesman ME, Hendrickson WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606.0193-4511(1996)272[1606:SAOSBB]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]