Abstract
Neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis have been termed protein misfolding disorders that are characterized by the neuronal accumulation of protein aggregates. Manipulation of the cellular stress-response involving induction of heat shock proteins (Hsps) in differentiated neurons offers a therapeutic strategy to counter conformational changes in neuronal proteins that trigger pathogenic cascades resulting in neurodegenerative diseases. Hsps are protein repair agents that provide a line of defense against misfolded, aggregation-prone proteins. These proteins are not induced in differentiated neurons by conventional heat shock. We have found that celastrol, a quinine methide triterpene, induced expression of a wider set of Hsps, including Hsp70B′, in differentiated human neurons grown in tissue culture compared to cultured rodent neuronal cells. Hence the beneficial effect of celastrol against human neurodegenerative diseases may exceed its potential in rodent models of these diseases.
INTRODUCTION
With the prevalence of neurodegenerative diseases on the rise as average life expectancy increases, the hunt for effective treatments and preventive measures for these disorders is a pressing challenge. Neurodegenerative disorders that differ widely in symptoms and patterns of neuron loss have been reported to involve aggregation-prone, misfolded proteins (Selkoe 2003; Forman et al 2004; Muchowski and Wacker 2005). Despite differences in primary amino acid sequence, signature disease proteins in a variety of these neural disorders share structural similarity in their resultant β-sheet–rich aggregates (Dobson 2001). It is unclear if these protein aggregates are causative agents of neuronal cell death or, on the contrary, act as a sequestering mechanism for the removal of toxic misfolded intermediates and soluble oligomers (Kayed et al 2003; Agorogiannis et al 2004; Ross and Poirier 2004). Nevertheless, confronted with a list of diverse neurodegenerative disorders, potential convergence of pathogenesis to the commonality of protein misfolding and aggregation suggests a shared target for treatment based on the function of heat shock proteins (Hsps) as protein repair agents that provide a line of defense against misfolded, aggregation-prone proteins (Muchowski and Wacker 2005).
Hsps have well-characterized roles in facilitating protein folding in de novo protein synthesis and during refolding of partially denatured proteins that arise after cellular stress (Morimoto et al 1997; Hartl and Hayer-Hartl 2002). They are a group of highly conserved and ubiquitous molecular chaperones conventionally subdivided into the following major families: Hsp110, Hsp90, Hsp70, Hsp60, Hsp40, and small Hsps. Their ability to recognize and bind to denatured or partially unfolded proteins allows Hsps to counter denaturation, misfolding, and irreversible aggregation of proteins. Family members have been implicated in the solubilization of aggregated proteins (Stege et al 1995). Because alteration of aggregation kinetics can affect the progression of the neurodegenerative disease (Wolozin and Behl 2000), upregulation of Hsps could alleviate neurodegeneration by modulating protein misfolding in affected neurons. This concept has led to a quest for pharmacological agents that can induce Hsps in neuronal cells as a therapeutic approach for combating neurodegeneration. Animal models of neurodegenerative diseases have demonstrated the beneficial effects of Hsp70 overexpression (Muchowski and Wacker 2005). Upregulation of a set of Hsps, rather than a single Hsp, will likely yield added benefits (Jana et al 2000; Patel et al 2005).
Celastrol has been identified as a potential neuroprotective candidate in a collaborative drug screen aimed at identifying therapeutic agents against neurodegenerative diseases from a panel of 1040 existing drugs, of which 75% are US Food and Drug Authority (FDA) approved (Abbott 2002; Heemskerk et al 2002). Using tissue culture–based assays, candidate drugs were scored on their ability to suppress aspects associated with neurodegenerative diseases such as protein aggregation. Celastrol, a quinine methide triterpene, is extracted from the vine Tripterygium wilfordii of the Celastraceae family (Zhou 1991). It has been used in traditional Chinese medicine for the treatment of various illnesses such as inflammation (Pinna et al 2004) and rheumatoid arthritis (Tao et al 2002). Celastrol has been reported to show antifertility effects on sperm (Yuan et al 1995; Bai et al 2003). Extracts from the plant also have been reported to exhibit immunosuppressive effects (Huang et al 1998).
To determine if celastrol induces Hsps when applied to neuronal cells, we employed two cell lines of different species origin, namely human (SH-SY5Y) and rodent (NG108-15) cells. The rationale for including these 2 neuronal cell lines was to explore species-specific differences in Hsp neuronal induction patterns that may influence the translation of observations on animal-based models of neurodegenerative diseases to the actual human condition. Celastrol treatment was carried out on the aforementioned cell lines under both undifferentiated and differentiated neuronal conditions. The differentiation status of neurons is of particular importance because differentiated neurons in both in vivo and in vitro systems have been reported to be refractory to Hsp induction following conventional heat shock (Manzerra et al 1993; Foster et al 1995; Dwyer et al 1996; Hatayama et al 1997; Batulan et al 2003). Our results indicate that celastrol induces expression of a wider set of potentially neuroprotective Hsps in differentiated human neurons compared to differentiated rodent neurons.
MATERIALS AND METHODS
Cell culture and induction of neuronal differentiation
The human SH-SY5Y cell line (American Type Culture Collection, Manassas, VA, USA) was maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The rodent NG108-15 cell line (American Type Culture Collection) was maintained in DMEM without sodium pyruvate supplemented with 10% FBS and sodium hypoxanthine, aminopterin and thymidine (HAT). Cultures were maintained at 37°C in a humidified 5% CO2 atmosphere. After plating and allowing for cell adhesion for 24 hours, neuronal differentiation of NG108-15 cells was induced by treatment with 1 mM N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (a cell-permeable cyclic adenosine monophosphate (cAMP) analog) for 72 hours at 37°C at reduced FBS (5%) before proceeding with dimethyl sulfoxide (DMSO) or celastrol treatment. Neuronal differentiation of SH-SY5Y cells was induced by treatment with 10 μM of all-trans-retinoic acid incubating at 37°C for 72 hours under serum free conditions. Celastrol was obtained from Gaia Chemical Corporation (Gaylordsville, CT, USA) and dissolved in DMSO as the vehicle.
Cell viability assays
Viability of undifferentiated NG108-15 and SH-SY5Y cells were examined by propidium iodide ([PI], Sigma, St Louis, MO, USA) exclusion methodology. PI was used as a viability dye (10 μg/ml), and 300 nM 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad CA, USA) as a counter stain for nuclei. Healthy cells that retain membrane integrity are stained blue by DAPI while being able to exclude PI. Dead cells (late apoptotic or necrotic) are PI positive due to loss of membrane integrity. Although being PI negative, early apoptotic cells also were scored by apoptotic nuclear morphology as stained by DAPI. A cell was scored as apoptotic if it displayed one or more of the following: nuclear margination, chromatin condensation, or formation of apoptotic bodies. Viability of differentiated NG108-15 and SH-SY5Y cells were examined by phase contrast microscopy. Healthy cells showed intact cell bodies with extended neurite processes. Dying cells showed extensive rounding of the cell body, with retraction and degeneration of neurites. Because differentiated cells rely on adhesion to the cultured surface for maintaining their differentiated morphology, we developed a cell death morphology assay that allows quantitative measurement of cellular viability of differentiated neuronal cells. Quantitative assay of the viability of differentiated NG108-15 and SH-SY5Y cells were performed by DAPI/PI costaining method on fixed cells. Cells were plated on coverslips and differentiation was induced as described above. Differentiated cells were fixed with 4% paraformaldehyde after 24 hours of treatment with celastrol followed by permeabilization with 0.1% Triton X-100. DAPI staining (300 nM) of the nucleus allowed for the observation of apoptotic nuclear morphology, whereas PI staining (10 μg/ml) and/or differential interference contrast (DIC) microscopy permitted the visualization of degenerating neurite morphology. Examples of viable and nonviable differentiated NG108-15 and SH-SY5Y cells are shown in Figure 3B. For each celastrol concentration point, 200 cells were counted in triplicate, and the data were expressed as the mean ± standard error (SE) of 3 independent experiments.
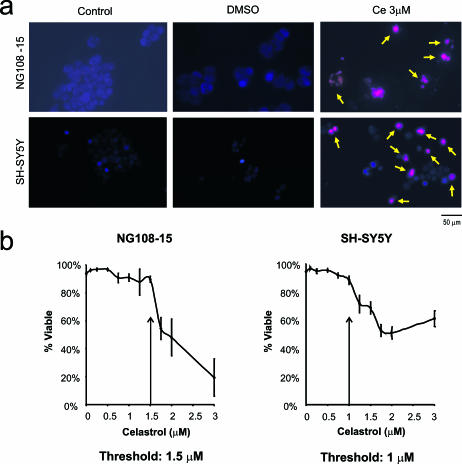
Fig 3.
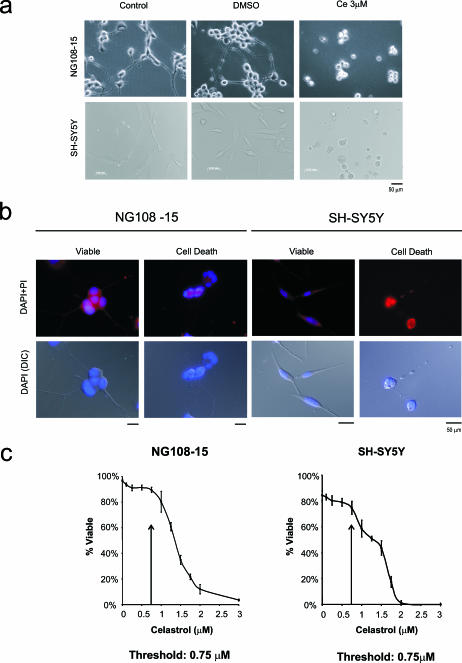
Effect of celastrol on differentiated neuronal cells. Microscopic analysis using (A) phase contrast microscopy and (B) 4′,6-diamidino-2-phenylindole (DAPI)/propidium iodide (PI) staining on fixed cells showed that celastrol at 3 μM induced cell death in differentiated human (SH-SY5Y) and rodent (NG108-15) neurons. (C) A dose-response analysis demonstrated that the threshold at which neuronal viability was affected was 0.75 μM for both human neurons and rodent neurons in the differentiated state
Western blotting analysis
After 24 hours of treatment with celastrol, cells were harvested and samples containing 30 μg of protein per lane were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Primary and secondary antibodies were added in 1% milk powder in phosphate-buffered saline (PBS) and incubated overnight at 4°C. Horseradish peroxidase-conjugated secondary antibody (Sigma) was detected by enhanced chemiluminescence assay (Amersham, Piscataway, NJ, USA). The following antibodies were used: Hsp25 (rodent isoform of Hsp27; SPA-801, Stressgen Biotechnologies, Victoria, BC, Canada); human Hsp27 (SPA-803, Stressgen); rodent Hsp32 (OSA-111, Stressgen); human Hsp32 (OSA-110, Stressgen); Hsp40 (SPA-400, Stressgen); Hsp70 (SPA-810, Stressgen); Hsp70B′ (SPA-754, Stressgen); Hsp110 (SPA-1101, Stressgen); constitutively expressed Hsc70 (SPA-815, Stressgen); and β-tubulin (MAB3408, Chemicon, Temecula, CA, USA). Western blots representative of 6 experimental repeats are shown.
RESULTS
Induction of Hsps in cultured human and rodent neurons by celastrol
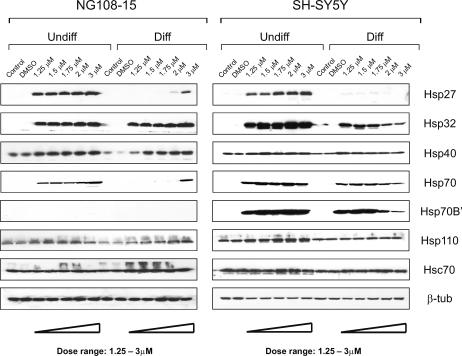
As shown in Figure 1, celastrol in the dosage range of 1.25 to 3 μM induced Hsps in both undifferentiated and differentiated human SH-SY5Y and rodent NG108-15 neuronal cells. A robust induction of Hsp27, Hsp32, and Hsp70 was apparent in undifferentiated cells. Following neuronal differentiation, the level of induction of Hsp27 and Hsp70 was reduced with less effect on Hsp32. Celastrol also caused a robust induction of an additional Hsp, namely Hsp70B′ (Leung et al 1990, 1992; Tavaria et al 1996) in both differentiated and undifferentiated human neurons. Induction of this Hsp was not observed in rodent neurons (Fig 1). To our knowledge, Hsp70B′ has not been studied previously in neural cells. Interestingly, as the dosage of celastrol was increased from 1.25 to 3 μM, a progressive decrease in the level of induction of Hsp27, Hsp32, Hsp70, and Hsp70B′ was apparent in the differentiated human neurons. This suggested that the high dosages of celastrol might be adversely affecting the cells, hence the next set of experiments were designed to address this point. Constitutively expressed Hsc70 was detected in both undifferentiated and differentiated human and rodent neurons and its level did not show marked changes after celastrol treatment. Induction of Hsps in the differentiated human neurons was impaired by higher dosages of celastrol without promoting degradation of constitutively expressed Hsc70.
Fig 1.
Induction of heat shock proteins (Hsps) in undifferentiated and differentiated human and rodent neurons by celastrol. Immunoblotting demonstrated a robust induction of Hsp27, Hsp32, and Hsp70 in undifferentiated (Undiff) human (SH-SY5Y) and rodent (NG108-15) neurons. Following neuronal differentiation (Diff), induction of Hsp27 and Hsp70 was reduced with less effect on Hsp32. Celastrol also triggered the induction of Hsp70B′, however, this was observed only in neurons of human origin. The constant level of the β-tubulin signal served as a control for equal loading of protein in each lane. The smear above the constitutively expressed Hsc70 band in the rodent blot represents a minor nonspecific band
Effect of celastrol on the cellular viability of undifferentiated neuronal cells
Microscopy revealed that celastrol at 3 μM influenced neuronal viability in undifferentiated human and rodent neuronal cells (Fig 2A). The propidium iodide exclusion methodology revealed a loss of membrane integrity after celastrol treatment, as indicated by the yellow arrows. Control cells were not affected (no treatment or DMSO vehicle alone). A dose-response analysis was undertaken to provide an estimate of cell death. This demonstrated that the threshold at which neuronal viability was affected was 1 μM for human neurons and 1.5 μM for rodent neurons in the undifferentiated state (Fig 2B).
Fig 2.
Effect of celastrol on the cellular viability of undifferentiated neuronal cells. (A) Microscopic analysis using propidium iodide (PI) exclusion methodology revealed that celastrol at 3 μM triggered cell death in undifferentiated human (SH-SY5Y) and rodent (NG108-15) neurons (indicated by arrows). Control, cells without treatment; dimethyl sulfoxide (DMSO), vehicle control for celastrol; Ce, celastrol dissolved in DMSO vehicle. (B) A dose-response analysis indicated that the threshold at which neuronal viability was affected was 1 μM for undifferentiated human neurons and 1.5 μM for undifferentiated rodent neurons
Effect of celastrol on differentiated neuronal cells
Differentiated human and rodent neurons exhibited extensive outgrowth of neurite processes (Fig 3A,B). These processes were lost after celastrol treatment at 3 μM, which coincided with cell shrinkage and rounding up of cell bodies (Fig 3A,B). A cell death assay was employed that allowed viable cell counts of differentiated neurons. The resultant dose-response analysis measuring both apoptotic nuclear morphology and neurite degeneration revealed that the threshold at which viability was affected was 0.75 μM for both human and rodent neuronal cells in the differentiated state (Fig 3C). The fact that highest dosages of celastrol induced Hsps in differentiated rodent neurons and not in differentiated human neurons (Fig 1) may be explained by the observation that some rodent cells survived the highest celastrol dosages, whereas human cells did not (Fig 3C).
Celastrol induces a wider set of Hsps in differentiated human neurons compared to differentiated rodent neurons
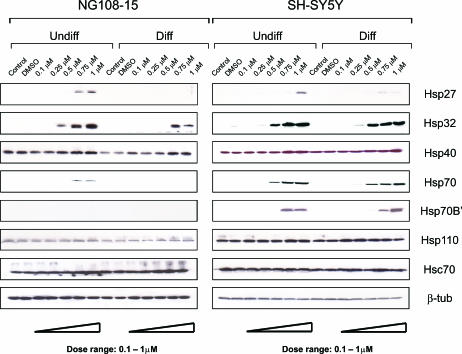
When dosages in the viable range were employed (Fig 4), celastrol was observed to induce a wider set of Hsps in differentiated human SH-SY5Y neurons compared to differentiated rodent NG108-15 neurons. For example, Hsp27, Hsp32, Hsp70, and Hsp70B′ were induced in differentiated human neurons at celastrol levels that did not affect viability, whereas induction of Hsp32 was observed in differentiated rodent neurons. Constitutively expressed Hsc70 and Hsp110 did not show marked changes.
Fig 4.
Celastrol induces a wider set of heat shock proteins (Hsps) in differentiated human neurons compared to differentiated rodent neurons. Human (SH-SY5Y) neurons and rodent (NG108-15) neurons were treated with celastrol at 0.1 to 1 μM with and without prior induction of differentiation. Immunoblotting revealed that dosages of celastrol within the viable range induced Hsp27, Hsp32, Hsp70, and Hsp70B′ in differentiated human neurons, and Hsp32 was induced in differentiated rodent neurons. Levels of constitutively expressed Hsc70 and Hsp110 did not show marked changes
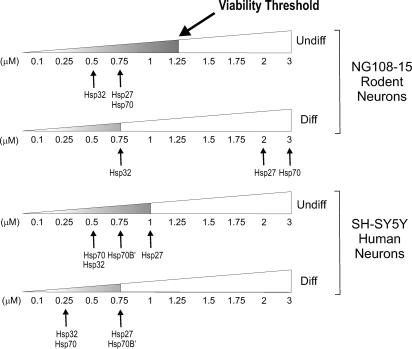
The minimal dosages of celastrol required to induce the various Hsps in both differentiated and undifferentiated human and rodent neurons are summarized in Figure 5. This clearly indicates that differentiated rodent neurons are not responsive to celastrol in terms of Hsp70 and Hsp27 expression at dosages in the viable range whereas Hsp27, Hsp32, Hsp70, and Hsp70B′ are responsive in the differentiated human neurons. Clearly celastrol induces this set of Hsps in the differentiated human neurons at viable dosages.
Fig 5.
Summary of minimal celastrol dosages required for induction of various Hsps in differentiated (Diff) and undifferentiated (Undiff) human and rodent neurons. Arrows indicate the minimal dosage of celastrol required for the induction of the corresponding Hsp. Celastrol treatments in the viable range are indicated by the shaded portion of the triangle that represents a dosage range of 0.1 to 3 μM celastrol
DISCUSSION
Neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis (ALS) have been termed protein-misfolding disorders that are characterized by the neuronal accumulation of protein aggregates (Muchowski and Wacker 2005). Manipulation of the cellular stress response involving induction of heat shock proteins in differentiated neurons offers a therapeutic strategy to counter conformational changes in neuronal proteins that trigger pathogenic cascades, resulting in neurodegenerative diseases. Hsps are protein repair agents that provide a line of defense against misfolded, aggregation-prone proteins. The ability of celastrol to induce a set of Hsps in differentiated human neuronal cells at dosages that do not affect neuronal viability qualifies it as a potential candidate for combating human protein misfolding neurodegenerative diseases using upregulation of Hsps as a therapeutic strategy. The differentiation status of neurons is of particular importance because differentiated neurons in both in vivo and in vitro systems have been reported to be refractory to Hsp induction following conventional heat shock (Manzerra et al 1993; Foster et al 1995; Dwyer et al 1996; Hatayama et al 1997; Batulan et al 2003). Hence the observed ability of celastrol to induce Hsps in differentiated human neurons is significant. Interestingly, our present study shows that celastrol is more limited in its ability to induce a set of Hsps in differentiated rodent neurons. Celastrol has been shown to induce Hsp70 via activation of heat shock transcription factor (HSF) 1 in undifferentiated neuroblastoma cells at 3 μM (Westerheide et al 2004), a dosage that our present studies suggest is detrimental to cell viability.
Animal models of Alzheimer's disease, Parkinson's disease, and ALS have been developed in rodents. Furthermore, therapeutic strategies for combating these neurodegenerative disorders, including the approach of upregulating Hsps, have led to testing of candidate drugs in these rodent models. Celastrol has been shown to be neuroprotective in rodent models of Parkinson's disease (Cleren et al 2005), Huntington's disease (Cleren et al 2005; Wang et al 2005), and ALS (Kiaei et al 2005). Our present studies on celastrol demonstrate that it induces a wider set of potentially neuroprotective Hsps in differentiated human neurons compared to differentiated rodent neurons, namely Hsp27, Hsp32, Hsp70, and Hsp70B′. Given this differential pattern of Hsp induction, celastrol may confer greater neuronal protective benefits against neurodegenerative disease in the human brain, exceeding its potential in the rodent-based neurodegenerative models mentioned above (Cleren et al 2005; Kiaei et al 2005; Wang et al 2005). It is unknown whether other human and rodent neuronal cell lines also would show that celastrol induces a wider set of Hsps in differentiated human neurons compared to differentiated rodent neurons or whether the difference in Hsp inducibility is unique to the particular cell lines used in this investigation. We specifically selected SH-SY5Y human cells and NG108-15 rodent cells for the present study because these cell lines have been used widely as tissue culture model systems for neurodegenerative diseases. SH-SY5Y cells have been employed as a model for the study of Parkinson's disease (Duka and Sidhu 2006; Ho et al 2006; Inden et al 2006) and Alzheimer's disease (Bandyopadhyay et al 2006; Bate et al 2006; Schaeffer et al 2006; Westmark et al 2006). NG108-15 cells have been used as a model for the study of Parkinson's disease (Pifl et al 1993), Alzheimer's disease (Yoshizaki et al 2004; Suga et al 2005; Tsukane et al 2007), and polyQ disorders (Ji et al 2006).
Our studies highlight the fact that the spectrum of Hsp70 genes present in the human genome includes members, such as Hsp70B′, that are not present in rodent genomes (Parsian et al 2000; Noonan et al 2007), and hence are not beneficial factors in animal models of neurodegenerative diseases to combat misfolded proteins. The Hsp70B′ gene appears to have arisen after the divergence of rodents and humans (Parsian et al 2000; Noonan et al 2007). Although Hsp70B′ and stress-inducible Hsp70 share 84% sequence identity, differences in the substrate-binding pocket and activation profiles may confer Hsp70B′ with a distinct cellular role (Noonan et al 2007). To our knowledge, Hsp70B′ has not received attention in the field of human neurodegenerative diseases. Noonan et al (2007) recently have stated that Hsp70B′ is a unique member of the human Hsp70 family of chaperones about which information is scarce. The present investigation demonstrates that celastrol induces Hsp70B′ in differentiated human neurons and contributes to the wider set of celastrol-inducible Hsps that is present in human neurons compared to rodent neurons. Our studies indicate that Hsp70B′ is strictly inducible and does not exhibit detectable basal expression in human neurons. In summary, celastrol is a promising candidate as a therapeutic agent for neurodegenerative diseases with the attractive feature of upregulating a wider set of Hsps in differentiated human neurons compared to rodent neurons.
Acknowledgments
This study was supported by grants from Natural Sciences and Engineering Research Council of Canada (NSERC) (I.R.B.) who also holds a Canada Research Chair.
REFERENCES
- Abbott A. Neurologists strike gold in drug screen effort. Nature. 2002;417:109. doi: 10.1038/417109a.1476-4687(2002)417[0109:NSGIDS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Agorogiannis EI, Agorogiannis GI, Papadimitriou A, Hadjigeorgiou GM. Protein misfolding in neurodegenerative diseases. Neuropathol Appl Neurobiol. 2004;30:215–224. doi: 10.1111/j.1365-2990.2004.00558.x.0305-1846(2004)030[0215:PMIND]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bai JP, Shi YL, Fang X, Shi QX. Effects of demethylzeylasteral and celastrol on spermatogenic cell Ca2+ channels and progesterone-induced sperm acrosome reaction. Eur J Pharmacol. 2003;464:9–15. doi: 10.1016/s0014-2999(03)01351-7.0014-2999(2003)464[0009:EODACO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Ni J, Ruggiero A, Walshe K, Rogers MS, Chattopadhyay N, Glicksman MA, Rogers JT. A high-throughput drug screen targeted to the 5′ untranslated region of Alzheimer amyloid precursor protein mRNA. J Biomol Screen. 2006;11:469–480. doi: 10.1177/1087057106287271.1087-0571(2006)011[0469:AHDSTT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bate C, Kempster S, Last V, Williams A. Interferon-gamma increases neuronal death in response to amyloid-beta1-42. J Neuroinflammation. 2006;3:7. doi: 10.1186/1742-2094-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batulan Z, Shinder GA, Minotti S, He BP, Doroudchi MM, Nalbantoglu J, Strong MJ, Durham HD. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci. 2003;23:5789–5798. doi: 10.1523/JNEUROSCI.23-13-05789.2003.0270-6474(2003)023[5789:HTFIOT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleren C, Calingasan NY, Chen J, Beal MF. Celastrol protects against MPTP- and 3-nitropropionic acid–induced neurotoxicity. J Neurochem. 2005;94:995–1004. doi: 10.1111/j.1471-4159.2005.03253.x.0022-3042(2005)094[0995:CPAMAN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dobson CM. The structural basis of protein folding and its links with human disease. Philos Trans R Soc Lond B Biol Sci. 2001;356:133–145. doi: 10.1098/rstb.2000.0758.1471-2970(2001)356[0133:TSBOPF]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Sidhu A. The neurotoxin, MPP+, induces hyperphosphorylation of Tau, in the presence of alpha-Synuclein, in SH-SY5Y neuroblastoma cells. Neurotox Res. 2006;10:1–10. doi: 10.1007/BF03033329.1029-8428(2006)010[0001:TNMIHO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Liu Y, Miao S, Bradley RJ. Neuronal differentiation in PC12 cells is accompanied by diminished inducibility of Hsp70 and Hsp60 in response to heat and ethanol. Neurochem Res. 1996;21:659–666. doi: 10.1007/BF02527722.0364-3190(1996)021[0659:NDIPCI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. doi: 10.1038/nm1113.1078-8956(2004)010[1055:NDADOD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Foster JA, Rush SJ, Brown IR. Localization of constitutive and hyperthermia-inducible heat shock mRNAs (hsc70 and hsp70) in the rabbit cerebellum and brainstem by nonradioactive in situ hybridization. J Neurosci Res. 1995;41:603–612. doi: 10.1002/jnr.490410506.0360-4012(1995)041[0603:LOCAHH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408.0193-4511(2002)295[1852:MCITCF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hatayama T, Takahashi H, Yamagishi N. Reduced induction of HSP70 in PC12 cells during neuronal differentiation. J Biochem (Tokyo) 1997;122:904–1010. doi: 10.1093/oxfordjournals.jbchem.a021851.0021-924X(1997)122[0904:RIOHIP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Heemskerk J, Tobin AJ, Bain LJ. Teaching old drugs new tricks. Meeting of the Neurodegeneration Drug Screening Consortium, 7–8 April 2002, Washington, DC, USA. Trends Neurosci. 2002;25:494–506. doi: 10.1016/s0166-2236(02)02236-1.0166-2236(2002)025[0494:TODNTM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ho PW, Chu AC, Kwok KH, Kung MH, Ramsden DB, Ho SL. Knockdown of uncoupling protein-5 in neuronal SH-SY5Y cells: effects on MPP+-induced mitochondrial membrane depolarization, ATP deficiency, and oxidative cytotoxicity. J Neurosci Res. 2006;84:1358–1366. doi: 10.1002/jnr.21034.0360-4012(2006)084[1358:KOUPIN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Huang FC, Chan WK, Moriarty KJ, Zhang DC, Chang MN, He W, Yu KT, Zilberstein A. Novel cytokine release inhibitors. Part I: Triterpenes. Bioorg Med Chem Lett. 1998;8:1883–1886. doi: 10.1016/s0960-894x(98)00331-x.0960-894X(1998)008[1883:NCRIPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Inden M, Taira T, and Kitamura Y. et al. 2006 PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson's disease rat model. Neurobiol Dis. 24:144–158. [DOI] [PubMed] [Google Scholar]
- Jana NR, Tanaka M, Wang G, Nukina N. Polyglutamine length–dependent interaction of Hsp40 and Hsp70 family chaperones with truncated N-terminal huntingtin: their role in suppression of aggregation and cellular toxicity. Hum Mol Genet. 2000;9:2009–2018. doi: 10.1093/hmg/9.13.2009.1460-2083(2000)009[2009:PLIOHA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ji JW, Yang HL, Kim SJ. Analysis of cag-8: a novel poly(Q)-encoding gene in the mouse brain. Biochem Biophys Res Commun. 2006;346:1254–1260. doi: 10.1016/j.bbrc.2006.06.029.0006-291X(2006)346[1254:AOCANP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Giabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469.0193-4511(2003)300[0486:CSOSAO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:246–254. doi: 10.1159/000090364.1660-2862(2005)002[0246:CBNCDA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Leung KC, Hall C, Rajendran M, Spurr NK, Lim L. The human heat shock genes HSPA6 and HSP7 are both expressed and localized to chromosome 1. Genomics. 1992;12:74–79. doi: 10.1016/0888-7543(92)90409-l.0888-7543(1992)012[0074:THHSGH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Leung TK, Rajendran MY, Monfries C, Hall C, Lim L. The human heat shock protein family. Expression of a novel heat-inducible HSP70 (HSP70B′) and isolation of its cDNA and genomic DNA. Biochem J. 1990;267:125–132. doi: 10.1042/bj2670125.0264-6021(1990)267[0125:THHSPF]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzerra P, Rush SJ, Brown IR. Temporal and spatial distribution of heat shock mRNA and protein (hsp70) in the rabbit cerebellum in response to hyperthermia. J Neurosci Res. 1993;36:480–490. doi: 10.1002/jnr.490360414.0360-4012(1993)036[0480:TASDOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat shock response: regulation and function of heat shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29.0071-1365(1997)032[0017:THSRRA]2.0.CO;2 [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587.1471-0048(2005)006[0011:MONBMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Rasoulpour RJ, Giardina C, Hightower LE. Cell number–dependent regulation of Hsp70B′ expression: evidence of an extracellular regulator. J Cell Physiol. 2007;210:201–211. doi: 10.1002/jcp.20875.0021-9541(2007)210[0201:CNROHE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Parsian AJ, Sheren JE, Tao TY, Goswami PC, Malyapa R, Van Rheeden R, Watson MS, Hunt CR. The human Hsp70B gene at the HSPA7 locus of chromosome 1 is transcribed but non-functional. Biochim Biophys Acta. 2000;1494:201–205. doi: 10.1016/s0167-4781(00)00203-7.0006-3002(2000)1494[0201:THHGAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Patel YJ, Payne Smith MD, de Belleroche J, Latchman DS. Hsp27 and Hsp70 administered in combination have a potent protective effect against FALS-associated SOD1-mutant-induced cell death in mammalian neuronal cells. Brain Res Mol Brain Res. 2005;134:256–274. doi: 10.1016/j.molbrainres.2004.10.028.0169-328X(2005)134[0256:HAHAIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pifl C, Giros B, Caron MG. Dopamine transporter expression confers cytotoxicity to low doses of the parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium. J Neurosci. 1993;13:4246–4253. doi: 10.1523/JNEUROSCI.13-10-04246.1993.0270-6474(1993)013[4246:DTECCT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna GF, Fiorucci M, Reimund JM, Taquet N, Arondel Y, Muller CD. Celastrol inhibits pro-inflammatory cytokine secretion in Crohn's disease biopsies. Biochem Biophys Res Commun. 2004;322:778–786. doi: 10.1016/j.bbrc.2004.07.186.0006-291X(2004)322[0778:CIPCSI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ross CA, Poirier MA 2004 Protein aggregation and neurodegenerative disease. Nat Med. 10(Suppl). S10–17. [DOI] [PubMed] [Google Scholar]
- Schaeffer V, Patte-Mensah C, Eckert A, Mensah-Nyagan AG. Modulation of neurosteroid production in human neuroblastoma cells by Alzheimer's disease key proteins. J Neurobiol. 2006;66:868–881. doi: 10.1002/neu.20267.0022-3034(2006)066[0868:MONPIH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264.1476-4687(2003)426[0900:FPIFW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stege GJ, Brunsting JF, Kampinga HH, Konings AW. Thermotolerance and nuclear protein aggregation: protection against initial damage or better recovery? J Cell Physiol. 1995;164:579–586. doi: 10.1002/jcp.1041640316.0021-9541(1995)164[0579:TANPAP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Suga K, Saito A, Tomiyama T, Mori H, Akagawa K. Syntaxin 5 interacts specifically with presenilin holoproteins and affects processing of betaAPP in neuronal cells. J Neurochem. 2005;94:425–439. doi: 10.1111/j.1471-4159.2005.03210.x.0022-3042(2005)094[0425:SISWPH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tao X, Younger J, Fan FZ, Wang B, Lipsky PE. Benefit of an extract of Tripterygium Wilfordii Hook F in patients with rheumatoid arthritis: a double-blind, placebo-controlled study. Arthritis Rheum. 2002;46:1735–1743. doi: 10.1002/art.10411.0004-3591(2002)046[1735:BOAEOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–28. doi: 10.1379/1466-1268(1996)001<0023:ahsgtt>2.3.co;2.1466-1268(1996)001[0023:AHGTTH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukane M, Yoshizaki C, Yamauchi T. Development and specific induction of apoptosis of cultured cell models overexpressing human tau during neural differentiation: implication in Alzheimer's disease. Anal Biochem. 2007;360:114–122. doi: 10.1016/j.ab.2006.10.003.0003-2697(2007)360[0114:DASIOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wang J, Gines S, MacDonald ME, Gusella JF. Reversal of a full-length mutant huntingtin neuronal cell phenotype by chemical inhibitors of polyglutamine-mediated aggregation. BMC Neurosci. 2005;6:1–12. doi: 10.1186/1471-2202-6-1.1471-2202(2005)006[0001:ROAFMH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, Bosman JD, Mbadugha BN, Kawahara TL, Matsumoto G, Kim S, Gu W, Devlin JP, Silverman RB, Morimoto RI. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200.0021-9258(2004)279[56053:CAIOTH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Westmark PR, Shin HC, Westmark CJ, Soltaninassab SR, Reinke EK, Malter JS. Decoy mRNAs reduce beta-amyloid precursor protein mRNA in neuronal cells. Neurobiol Aging. 2006;27:787–796. doi: 10.1016/j.neurobiolaging.2006.03.003.0197-4580(2006)027[0787:DMRBPP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wolozin B, Behl C. Mechanisms of neurodegenerative disorders: Part 1: protein aggregates. Arch Neurol. 2000;57:793–796. doi: 10.1001/archneur.57.6.793.0003-9942(2000)057[0793:MONDPP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yoshizaki C, Tsukane M, Yamauchi T. Overexpression of tau leads to the stimulation of neurite outgrowth, the activation of caspase 3 activity, and accumulation and phosphorylation of tau in neuroblastoma cells on cAMP treatment. Neurosci Res. 2004;49:363–371. doi: 10.1016/j.neures.2004.04.005.0168-0102(2004)049[0363:OOTLTT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yuan YY, Gu ZP, Shi QX, Qin GW, Xu RS, Cao L. In vitro inhibition of celastrol on spermatozoa fertilization ability of guinea pig. Yao Xue Xue Bao. 1995;30:331–335.0513-4870(1995)030[0331:IVIOCO]2.0.CO;2 [PubMed] [Google Scholar]
- Zhou BN 1991 Some progress on the chemistry of natural bioactive terpenoids from Chinese medicinal plants. Mem Inst Oswaldo Cruz. 86(Suppl). 2:219–226. [DOI] [PubMed] [Google Scholar]