Abstract
Diapause-destined embryos of the crustacean Artemia franciscana arrest as gastrulae, acquire extreme stress tolerance, and enter profound metabolic dormancy. Among genes upregulated at 2 days postfertilization in these embryos is a homologue of p8, a stress-inducible transcription cofactor. Artemia p8 is smaller than vertebrate homologues but shares a basic helix-loop-helix domain and a bipartite nuclear localization signal. Probing of restriction digested DNA on Southern blots indicated a single Artemia p8 gene and 5′-RACE specified 2 transcription start sites. Several putative cis-acting regulatory sequences, including two heat shock elements, appeared upstream of the p8 transcription start site. Artemia p8 mRNA increased sharply at 1 day postfertilization in diapause-destined embryos and then declined, whereas p8 protein appeared 2 days postfertilization and remained relatively constant throughout development, indicating a stable protein. p8 was not detectable in nauplius-destined (nondiapause) Artemia embryos. Immunofluorescent staining revealed p8 within Artemia nuclei. The results support the idea that p8, a known stress-responsive transcription cofactor, mediates gene expression in diapause-destined Artemia embryos. p8 is the first diapause-related transcription factor identified in crustaceans and 1 of only a small number of such proteins identified in any organism undergoing diapause.
INTRODUCTION
Diapause, which occurs in many different organisms, is characterized by reduced cell growth, inhibition of development, and enhanced stress resistance (Denlinger 2002; Lopes et al 2004; MacRae 2005; Baumeister et al 2006; Koštál 2006), properties that are dependent on differential gene expression. As 1 example, embryos of the crustacean Artemia franciscana undergo diapause, arresting as gastrulae, encysting, and entering a deep state of metabolic dormancy (MacRae 2003). The encysted embryos (cysts) are extremely stress tolerant (Clegg 1997; Clegg et al 2000), a property thought to depend on their rigid chitinous walls (Anderson et al 1970), limited consumption of energy stores (Clegg 1997; Clegg and Jackson 1998), trehalose (Viner and Clegg 2001), and molecular chaperones (Sun et al 2004, 2006; Sun and MacRae 2005; Qiu et al 2006; Villeneuve et al 2006; Chen et al 2007). Artemia cysts and their postdiapause development are relatively well characterized, but little is known about the molecular events modulating cell growth and metabolism during diapause.
In work to be reported separately, subtractive hybridization was employed to identify upregulated genes in Artemia embryos undergoing diapause. Among these was the gene for a homologue of mammalian p8, also called candidate of metastasis-1 (com 1) (Ree et al 1999; Bratland et al 2000), first observed in rat acinar cells because its synthesis is enhanced during acute pancreatitis (Mallo et al 1997). p8 possess a basic helix-loop-helix motif characteristic of DNA binding proteins (Massari and Murre 2000; Jones 2004) and a bipartite, nuclear targeting signal (Mallo et al 1997; Ree et al 1999; Vasseur et al 1999a; Igarashi et al 2001; Valacco et al 2006), reflecting its localization within nuclei (Goruppi and Kyriakis 2004; Jiang et al 2005, 2006; Valacco et al 2006). p8 shares properties with the high mobility group (HMG) proteins even though sequence similarity is low and the AT hook motif is absent. Like HMG proteins, p8 is mainly random coil or unstructured in solution and binds DNA in a sequence-independent manner, an activity enhanced by protein kinase A–mediated phosphorylation and resulting in protein structural stabilization (Encinar et al 2001; Malicet et al 2006a). p8 is a transcription cofactor and it influences transforming growth factor β-1 (TGFβ-1) activation of the Smad transcription factor (Garcia-Montero et al 2001). p8 also activates the glucagon gene by promoting Pax2A and Pax2B function, in part by recruitment of p300 (Hoffmeister et al 2002), and it may modulate luteinizing hormone β (LHβ) gene expression in the gonadotrope as a stage-specific transcriptome member with an architectural role (Quirk et al 2003).
To summarize, Artemia p8 shares extensive sequence similarity with this protein from other organisms and, although smaller in size due to an amino-terminal deletion, Artemia p8 is a basic helix-loop-helix transcription cofactor with a bipartite nuclear localization signal. Artemia embryos developing directly into nauplii fail to express p8, whereas diapause-destined embryos exhibit a dramatic postfertilization increase in p8 mRNA, followed by appearance of the corresponding protein. p8 mRNA declines during embryo development, reaching very low levels prior to cyst release, but the protein remains and is found in postdiapause cysts where it localizes to nuclei. This pattern of synthesis, in concert with nuclear localization, suggests p8 influences development of diapause-destined Artemia embryos. This is the first indication that p8, a stress-associated transcription cofactor known to modulate cell growth, division, and apoptosis, influences gene expression in organisms negotiating the physiological processes characteristic of diapause.
MATERIALS AND METHODS
Artemia culture
A. franciscana cysts (INVE Aquaculture, Inc., Ogden, UT, USA) were hydrated overnight in distilled water at 4°C, rinsed 3 times with water, and either homogenized or incubated at 27°C for 20 hours with shaking at 200 RPM in hatch medium (Langdon et al 1990). Synchronous emerged nauplii (E2) were either homogenized or allowed to develop into instar I and II larvae (Liang and MacRae 1999). Adult Artemia were maintained at room temperature in filtered, aerated seawater and embryos were collected at 1-day intervals postfertilization (Qiu et al 2006).
p8 cDNA cloning
Full-length p8 cDNA was generated by 5′- and 3′-RACE using primers based on a partial p8 sequence obtained by subtractive hybridization. For 5′-RACE, total RNA from diapause-destined embryos 2 days postfertilization was prepared with TRIzol (Invitrogen, Burlington, ON, Canada). Ten micrograms of RNA were incubated with calf intestine alkaline phosphatase (Ambion, Inc., Austin, TX, USA) at 37°C for 1 hour and purified with acid phenol:chloroform. The RNA then was treated with tobacco acid pyrophosphatase (Ambion) at 37°C for 1 hour, ligated to the 5′-RACE adaptor (5′-GCUGAUGGCGAUGAACACUGCGUUUGCUGGCUUUGAUGAAA-3′) using T4 RNA ligase (Ambion), and reverse transcribed at 42°C for 1 hour in a mixture containing random decamers, M-MLV reverse transcriptase (Ambion), and dNTP. Nested polymerase chain reaction (PCR) was performed with p8 outer primer (5′-CACCAAGAGCCCTACATGTTGCTA-3′), p8 inner primer (5′-TCGGAAGTCCGGGACCTATAGAAT-3′), adaptor outer primer (5′-GCTGATGGCGATGAATGAACACTG-3′), and adaptor inner primer (5′-CGCGGATCCGAACACTGCGTTTGCTGGCTTTGATG-3′). For 3′-RACE, the p8 outer (5′-AGGTAGTAGGATCAGGTGTGACGA-3′) and inner primers (5′-ACCCTAGTGGACACTCAAAGAAAGC-3′), and adaptor outer (5′-GCGAGCACAGAATTAATACGACT-3′) and inner primers (5′-CGCGGATCCGAATTAATACGACTCACTCACTATAGG-3′) were used. RACE PCR conditions were 94°C for 3 minutes then 35 cycles of 94°C for 30 seconds, 60°C for 30 seconds, 72°C for 1 minute, followed by 72°C for 7 minutes. The PCR products were inserted into the TA cloning vector pCR2.1 (Invitrogen) and used to transform TOPO10 competent Escherichia coli (Invitrogen) prior to sequencing (DNA Sequencing Facility, Centre for Applied Genomics, Hospital for Sick Children, Toronto, ON, Canada).
To obtain full-length p8 cDNA, RNA was prepared from diapause-destined embryos at 2 days postfertilization and genomic DNA was eliminated with the TURBO DNA-free kit (Ambion). RT-PCR was performed with Pfu DNA polymerase (Fermentas Life Sciences, Burlington, ON, Canada); forward primer (5′-GCGGATCCATGTCAGAAGATCATTTTGATA-3′), including a BamHI site; and reverse primer (5′-CGCTGCAGAGTCATTTTTTGTCAGCACG-3′), including a PstI site. PCR was at 95°C for 2 minutes, followed by 30 cycles of 95°C for 1 minute, 56°C for 45 seconds, and 72°C for 1 minute, with extension at 72°C for 5 minutes. After addition of adenosine with the A-Addition kit (Qiagen, Mississauga, ON, Canada), RT-PCR products were inserted into the T/A cloning vector pCR2.1 (Invitrogen), followed by transformation of TOPO10 E. coli (Invitrogen). Plasmid DNA was digested with BamHI and PstI. cDNA inserts purified with the GFX PCR and Gel Band Purification Kit (Amersham Bioscience, Baie d'Urfe, Quebec, Canada), were ligated into the His-tagged pRSET A vector (Invitrogen) and transformed into TOP 10 F' E. coli (Invitrogen).
Artemia p8 gene
p8 cDNA was labeled with digoxigenin-11-dUTP by using the PCR Dig Labeling Mix (Roche, Mannheim, Germany) with the forward primer (5′-GCGGATCCATGTCAGAAGATCATTTTGATA-3′) and the reverse primer (5′-CGCTGCAGAGTCATTTTTTGTCAGCACG-3′). PCR conditions were 95°C for 2 minutes followed by 35 cycles of 95°C for 1 minute, 60°C for 30 seconds, and 72°C for 45 seconds. The products were purified with the GFX PCR and Gel Band Purification Kit (Amersham Bioscience). Fifteen-microgram samples of genomic DNA (Qiu et al 2006) were digested with XhoI, PstI, SalI, BamHI, and HindIII and then precipitated with ethanol, dissolved in 20 μL of TE buffer, and electrophoresed in 0.7% agarose. Before DNA transfer to nylon membranes, the gels were immersed twice in denaturation solution (0.5 M NaOH, 1.5 M NaCl) for 15 minutes, followed by two 15-minute incubations in neutralization solution (0.5 M Tris-HCl, pH 7.5, 1.5 M NaCl) and 10 minutes in 20 × standard saline citrate ([SSC], 0.3 M C6H5Na3O7, 3.0 M NaCl, pH 7.0). Membranes were washed in 2 × SSC, baked at 80°C for 2 hours, prehybridized in Dig Easy Hyb (Roche) at 42°C for 6 hours, and hybridized with probes overnight at 43°C. Membranes were washed twice at room temperature in 2 × SSC containing 0.1% sodium dodecyl sulfate (SDS) and twice at 68°C in 0.5 × SSC containing 0.1% SDS and stained with CDP-Star (Roche).
Genomic DNA digested overnight at 37°C with Sau3AI, SalI, EcoRI, HindIII, PstI, and XbaI, and the fragments were ligated at 16°C for 30 minutes into cassettes designed for LA PCR in vitro cloning (TaKaRa, Otsu, Japan). Nested PCR was performed with primers C1 (5′-GTACATATTGTCGTTAGAACGCGTAATACGACTCA-3′), S1 (5′-CAGCCTTCCACTTTGCCCGGAGAAGAGATG-3′), C2 (5′-CGTTAGAACGCGTAATACGACTCACTATAGGGAGA-3′), and S2 (5′-TTGTCCATATCAAAGTTAAAGTGTTCAAATCTATC-3′). C1 and C2 were based on cassette sequence, whereas S1 and S2 were designed on p8 cDNA sequence. Reaction conditions were 35 cycles of 94°C for 30 seconds, 60°C for 1 minute, 72°C for 2 minutes, followed by 72°C for 5 minutes. PCR products were purified with the GFX PCR DNA and Gel Band Purification Kit (Amersham Biosciences), cloned into the pGEM-T Easy vector (Promega, Madison, WI, USA), sequenced, and analyzed for cis-acting sites (www.genomatrix.de; Cartharius et al 2005).
p8 mRNA quantification
For quantification of p8 transcripts RNA prepared at daily intervals from Artemia embryos was reverse transcribed and quantitative PCR was performed with the iCycler (Bio-Rad, Mississauga, ON, Canada) in 25-μL mixtures containing 0.5 μL of cDNA, 12.5 μL of Platinum SYBR Green qPCR Supermix-UDG (Invitrogen), 0.5 μL of Rox reference dye (Invitrogen), 50 ng of forward primer 5′-AGGTAGTAGGAT CAGGTGTGACGA-3′, and 50 ng of reverse primer 5′-TCGGAAGTCCGGGACCTATAGAAT-3′. Reaction conditions were 50°C for 2 minutes, 95°C for 2 minutes, 45 cycles of 95°C for 15 seconds, 55°C for 30 seconds, 72°C for 30 seconds, followed by extension at 72°C for 5 minutes. α-tubulin mRNA was amplified as internal control using forward primer 5′-CTGCATGCTGTACAGA GGAGATGT-3′ and reverse primer 5′-CTCCTTCAAGAGAGTCCATGCCAA-3′ (Qiu et al 2006).
Antibody production and p8 detection
E. coli BL21 (DE3) pLysS (Invitrogen) transformed with p8 cDNA were incubated in 1 mM isopropyl β-D-thiogalactopyranoside ([IPTG], Sigma, Oakville, ON, Canada) for 6 hours. p8 protein was purified with BD TALON™ (BD Biosciences Clontech, Mississauga, ON, Canada; Sun et al 2004) and emulsified with TiterMax™ Gold Adjuvant (Sigma). Rabbits from Charles River Canada (St. Constant, Quebec, Canada), cared for in accordance with Guide to the Care and Use of Experimental Animals, available from the Canadian Council on Animal Care, were injected 3 times at 15-day intervals with purified p8. Serum was harvested 45 days after the first injection.
Artemia embryos collected at daily intervals postfertilization were homogenized in TRIzol (Invitrogen) and the homogenate was incubated for 5 minutes at room temperature. Eighty microliters of chloroform were added and the mixture was centrifuged at 12 000 × g for 15 min at 4°C. The upper protein-containing layer was mixed with 120 μL of ethanol, incubated at room temperature for 3 minutes, and centrifuged at 2000 × g for 5 minutes at 4°C. The supernatant was incubated with 600 μL of isopropyl alcohol for 10 minutes at room temperature and centrifuged at 12 000 × g for 10 minutes at 4°C. The pellet was washed 3 times in 95% ethanol, dissolved in 30 μL of 1% SDS, mixed with 4 × treatment buffer, and electrophoresed in 12.5% SDS polyacrylamide gels prior to staining with Coomassie blue or blotting to nitrocellulose (Bio-Rad, Herculus, CA, USA). Blots were incubatedwith antibody to p8 followed by horseradish peroxi-dase– (HRP) conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, Mississauga, ON, Canada; Liang et al 1997). Immunoconjugates were detected with Western Ligthning Enhanced Chemiluminescence (ECL) Reagent Plus (PerkinElmer Life Sciences, Boston, MA, USA).
Immunostaining of Artemia nuclei
Nuclei purified from cysts and instar II larvae (Sun et al 2004) were placed on poly-L-lysine–coated slides, fixed in 4% (W/V) paraformaldehyde for 20 minutes, hydrated in phosphate-buffered saline ([PBS], 140 mM NaCl, 2.7 mM KCl, 8.0 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4) for 5 minutes, and incubated with p8 antibody for 30 minutes at room temperature. The samples were washed 3 minutes with PBS containing 0.5% bovine serum albumin (Sigma) and 0.75% Triton X-100 ([PBSAT], Sigma), followed by incubation for 30 minutes with fluorescein isothiocyanate– (FITC) conjugated goat anti-rabbit IgG secondary antibody (Jackson ImmunoResearch). The samples were washed 3 times with PBS for 3 minutes, incubated with 0.001 μg/mL 4′6-diamidino-2-phenylindole dihydrochloride (DAPI) for 5 minutes, washed with water for 5 minutes, and mounted in 0.2 M 1,4-diazabicyclo[2.2.2]octane (DABCO) in 80% glycerol. Slides were examined by epifluorescence and confocal microscopy.
RESULTS
Artemia p8 sequence and domain structure
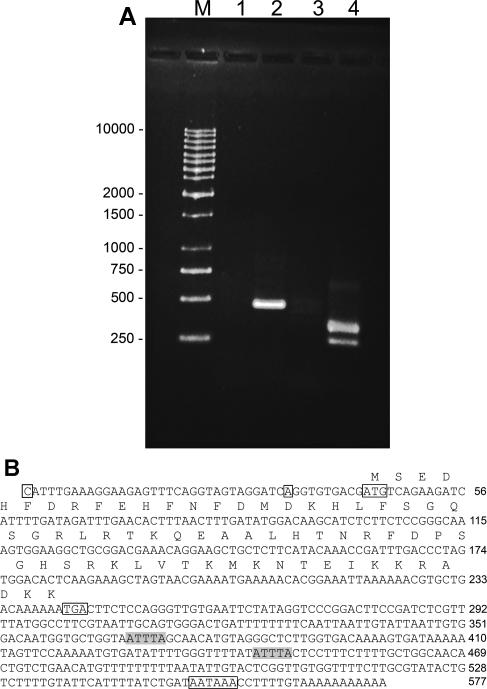
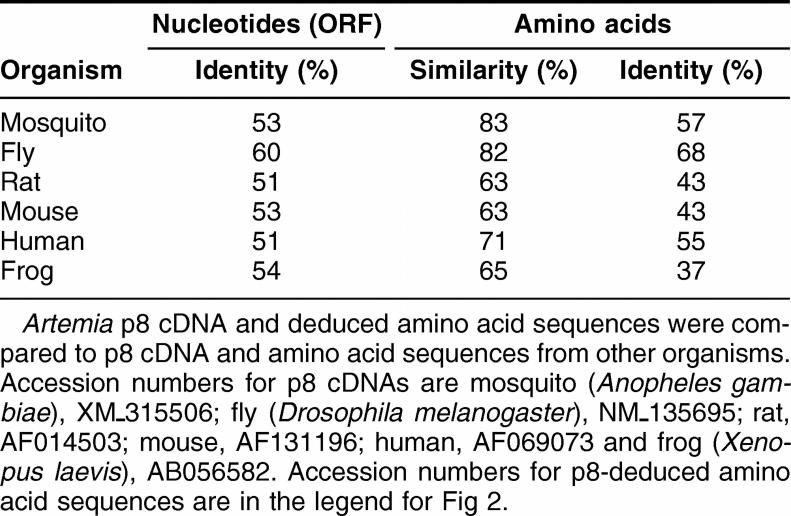
A partial p8 cDNA sequence was obtained by subtractive hybridization in experiments designed to identify genes upregulated in diapause-destined Artemia embryos. The cDNA was extended in both directions, yielding a 480 bp product via 3′-RACE and DNA fragments of 240 and 273 bps for the 5′-reaction, the latter suggesting 2 transcription start sites (Fig 1). Full length p8 cDNA generated by RT-PCR contained an open reading frame of 198 bps and a 5′-noncoding sequence of 43 bps, with transcription start sites 10 and 43 bps upstream of the translation start site. The 336 bp 3′-noncoding region included 2 ATTTA sequences indicative of rapidly degraded mRNAs, a polyadenylation signal of AATAAA, and a poly(A) tail. As determined by the use of GeneRunner software the deduced amino acid sequence of 66 residues has a molecular mass of 7.9 kDa and a pI of 9.90. Artemia p8 was most similar to p8 from Drosophila melanogaster and Anopheles gambiae, although sequence similarity was high, especially in the carboxy half of the protein, for all species examined (Fig 2; Table 1). Artemia p8 contained a basic helix-loop-helix DNA binding domain consisting of the conserved peptides, basic, 26-GRLRTK-31, helix I, 32-QEAALHT-38, loop, 39-NRFDPSG-45, and helix II 46-HSRKLVTKM-54 (Fig 2). A bipartate nuclear localization sequence encompassing residues R48 to D64 overlapped with helix II (Fig 2). Only p8 from mosquito was shorter than Artemia p8 and, in comparison to vertebrates, p8 from invertebrates lacked an amino terminal peptide of 18 or more amino acid residues. The amino-terminal region was more variable than the basic helix-turn-helix domain.
Fig 1.
Cloning and sequencing of Artemia p8. (A) RNA from diapause destined embryos was extended by 3′- and 5′-RACE. DNA products were electrophoresed in agarose gels and stained with Gel Star. Lane 1: 3′-RACE lacking cDNA; lane 2: 3′-RACE; lane 3: 5′-RACE lacking cDNA; lane 4: 5′-RACE; M, size markers in bp. (B) The complete p8 cDNA and deduced amino acid sequences. Boxed C and A, transcription start sites; boxed ATG, translation start site; boxed TGA, stop codon; boxed AATAAA, polyadenylation signal; shaded ATTTA, sequences indicative of rapidly degraded mRNAs
Fig 2.
p8 sequence comparison. p8 amino acid sequences from mosquito (XP_315506), fly (NP_609539), rat (AAB94673), mouse (NP_062712), human (AAC19384), and frog (BAB33387) were compared by ClustalW to Artemia (shrimp) p8. Asterisk (*), identical residue; colon (:), conserved substitution; period (.), semi-conserved substitution. Basic, helix I, loop, and helix II motifs are boxed; NLS, nuclear localization signal with key basic residues shaded in Artemia p8
Table 1.
p8 sequence comparisons
The Artemia p8 gene and putative cis-acting regulatory elements
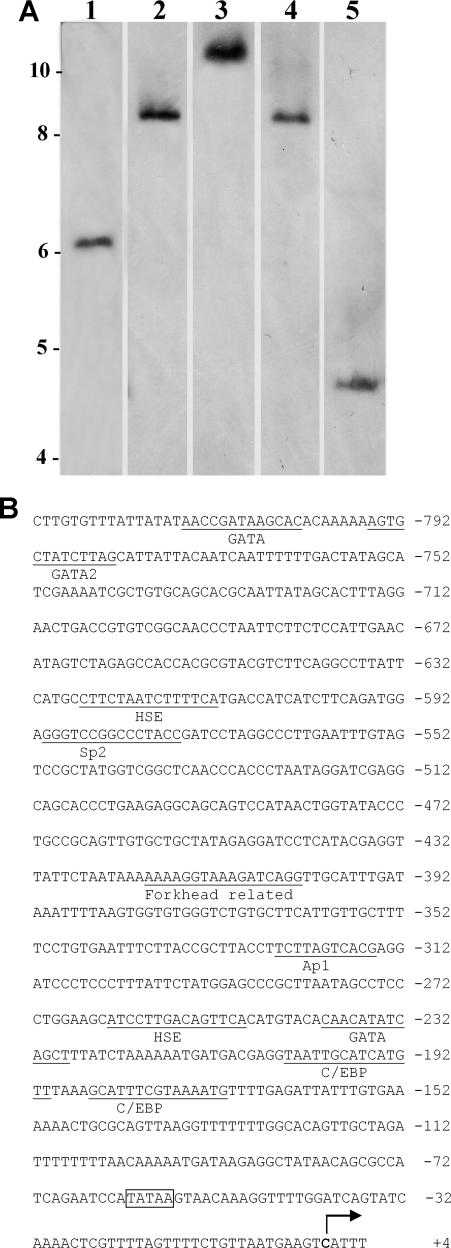
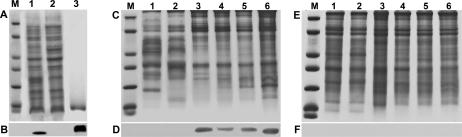
Southern blots probed with full-length p8 cDNA yielded 1 band for each restriction enzyme tested, suggesting a single p8 gene in Artemia (Fig 3A). LA PCR in vitro cloning generated 831 bp of DNA sequence upstream of the p8 gene. This region contained a TATA box 57 bp from the transcription start site and putative cis-acting transcription regulatory sites such as GATA2, Sp2, AP-1, CEBP, and heat shock elements (Fig 3B).
Fig 3.
Artemia p8 gene. (A) Digested Artemia genomic DNA was electrophoresed in agarose, transferred to nylon membranes, and probed with p8 cDNA. Lanes 1–5: loaded with DNA digested, respectively, with XhoI, PstI, SalI, BamHI and HindIII. Size in kbp is indicated on the left. (B) Upstream sequence of the p8 gene obtained by LA polymerase chain reaction (PCR) in vitro cloning. Boxed TATAA, TATA box; bold C with arrow, transcription start site; several putative cis-acting regulatory sites are underlined and labeled
p8 mRNA and protein in Artemia embryos
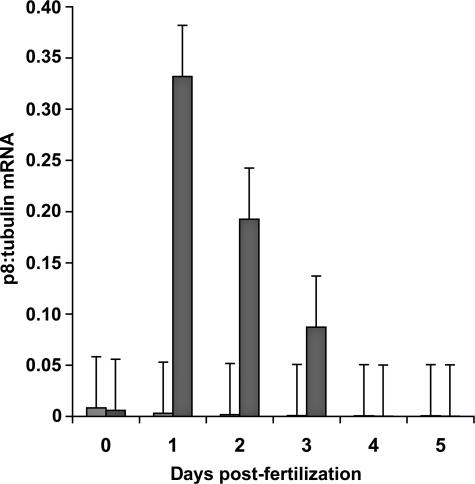
p8 mRNA increased markedly by day 1 postfertilization in diapause-destined Artemia embryos and then decreased until barely detectable at days 4 and 5, whereas embryos developing directly into larvae exhibited only minor traces of p8 transcript (Fig 4). For example, day 1 diapause-destined embryos contained approximately 60-fold more p8 mRNA than embryos developing into larvae. Probing Western blots of protein extracts from Artemia embryos with antibody generated to affinity purified, bacterially produced p8 (Fig 5A,B) revealed p8 initially in diapause-destined embryos 2 days postfertilization and, although staining intensity varied, the protein persisted until day 5 (Fig 5C,D). p8 was absent from embryos undergoing development into nauplii (Fig 5E,F). The immunoprobing of Western blots corroborated the demonstration of differential gene expression obtained by quantitative PCR, although the p8 protein was more stable than mRNA.
Fig 4.
p8 mRNA during embryo development. p8 mRNA from Artemia embryos was measured at daily intervals postfertilization by quantitative polymerase chain reaction (PCR) using Artemia α-tubulin mRNA as standard. Light and dark shaded bars, p8 mRNA in nauplii and diapause-destined embryos, respectively
Fig 5.
Antibody production and p8 detection. p8 synthesized in transformed Escherichia coli was purified on TALON affinity resin for use as antigen. Protein samples were electrophoresed in sodium dodecyl sulfate (SDS) polyacrylamide gels and either stained with Coomassie blue (A) or transferred to nitrocellulose and detected with Omniprobe (B). Lane 1: extract from bacteria transformed with p8 cDNA; lane 2: extract from bacteria transformed with vector only; lane 3: purified p8. Lanes 1 and 2: 30 μg of protein in A and 10 μg in B; lane 3: 3 μg of protein in A and 0.4 μg in B. Protein extracts prepared from diapause-destined (C, D) and nauplii-destined (E, F) Artemia embryos at daily intervals postfertilization, were electrophoresed in SDS polyacrylamide gels and either stained with Coomassie blue (C, E) or transferred to nitrocellulose and probed with antibody to p8 (D, F). Lanes 1–6: day 0 (fertilization) to day 5 postfertilization, respectively. All lanes received 30 μg of protein. M, molecular mass markers of 116.0, 66.2, 45.0, 35.0, 25.0, 18.4, and 14.4 kDa
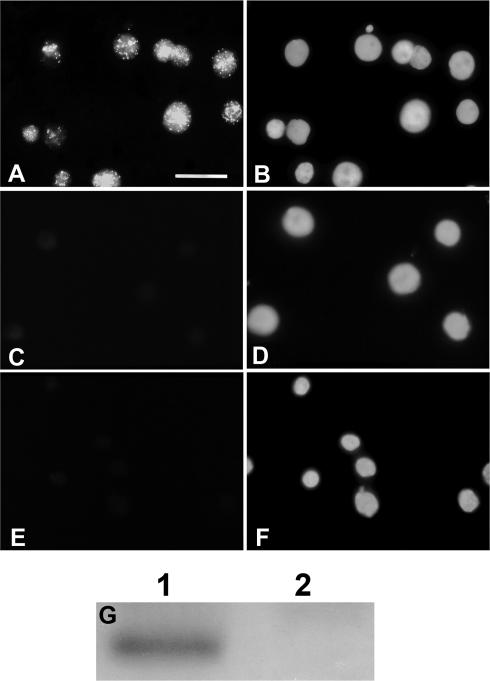
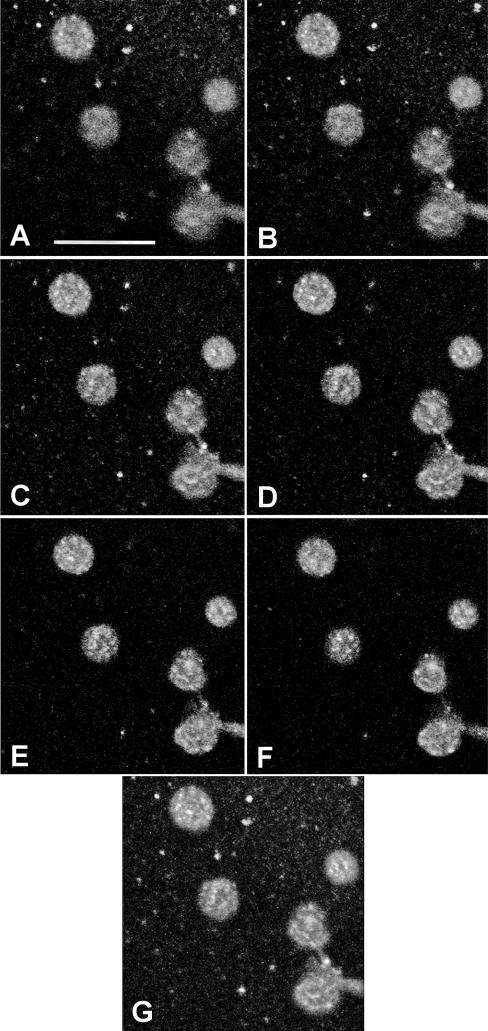
Localization of Artemia p8
p8 was distributed in a speckled pattern throughout Artemia cyst nuclei (Figs 6, 7). Instar II larvae nuclei, which lacked sufficient p8 to be detectable on Western blots, had no staining.
Fig 6.
Immunolocalization of Artemia p8. Nuclei from Artemia cysts (A, B) and instar II larvae (C, D) were incubated with antibody to p8 followed by fluorescein isothiocyanate– (FITC) conjugated goat anti-rabbit IgG and 4′6-diamidino-2-phenylindole dihydrochloride (DAPI). Cyst nuclei (E, F) were incubated in buffer lacking primary antibody followed by secondary antibody and DAPI. (A, C, E) FITC; (B, D, F) DAPI. The bar in A represents 20 μm and all images are the same magnification. (G) Equivalent amounts of protein from cysts (1) and larvae (2) were electrophoresed in SDS polyacrylamide gels, transferred to nitrocellulose, and probed with antibody to p8
Fig 7.
p8 distribution in Artemia nuclei. Cyst nuclei stained with antibody to p8 and fluorescein isothiocyanate– (FITC) conjugated goat anti-rabbit IgG were examined by confocal microscopy. (A–F) Serial optical sections of nuclei; (G) reconstruction of sections. The bar in A represents 25 μm and all magnifications are the same
DISCUSSION
Molecular analysis of diapause induction and the ensuing events that modulate cell growth and stress resistance in Artemia embryos yielded a developmentally regulated basic helix-loop-helix transcription cofactor termed p8 (Igarashi et al 2001; Valacco et al 2006). This protein from Artemia and other invertebrates lacks several amino terminal residues constituting a PEST region in vertebrate p8, which is enriched in Pro, Glu, Ser, and Thr and thought to promote ubiquitin-dependent protein degradation by proteasomes (Goruppi and Kyriakis 2004). Susceptibility to ubiquitin-mediated digestion, as indicated for breast cancer cell p8 (Jiang et al 2005), regulates protein longevity and hence activity. That Artemia p8 persists into postdiapause development may depend on the absence of the PEST region, allowing the protein to affect gene expression for an extended period. In marked dissimilarity, p8 mRNA half-life is much shorter, perhaps influenced by ATTTA sequences in the 3′-untranslated region, these normally associated with rapidly degraded transcripts (Moribe et al 2001).
Humans and mice each contain a single p8 gene with the 5′-flanking region of the mouse gene displaying a TATA box equivalent, as well as C/EBP, SBE, Myc, Sp1, NFkB, and Ap1 binding sites (Vasseur et al 1999b). Overlapping C/EBP and SBE sites are important cis-acting elements for the mouse p8 gene with the CAAT-enhancer binding proteins C/EBPα and C/EBPβ able to promote p8 gene transcription in NIH 3T3 cells (Vasseur et al 1999b; García-Montero et al 2001). The 5′-untranslated region of the human p8 gene lacks authentic proximal TATA and CAAT boxes (Vasseur et al 1999a), with the CAAT box also missing from the Artemia p8 gene. Expression of the LHβ gene in mouse pituitary-derived gonadotrope cell lines may require p8 synthesis, which is proposed to depend on the transcription factor GATA2 (Quirk et al 2003). In comparison to the situation in mammals, Artemia possess 1 p8 gene, although in apparent contrast to mice and humans, there are 2 transcription start sites for the single open reading frame (ORF). The upstream region of the Artemia p8 gene contains a TATA box and several putative cis-acting regulatory sequences, including 2 heat shock elements (HSE) of particular interest from a stress induction perspective, and Sp2, Ap1, GATA2, and C/EBP sites, all requiring further analysis to determine their regulatory significance.
Expression patterns and nuclear localization corroborate the proposal that p8 regulates gene expression during Artemia diapause, the first example of a crustacean protein with this function. A handful of diapause-related transcription factors are documented with DAF-12 and DAF-16/FOXO, modulators of Caenorhabditis elegans diapause (dauer) (Baumeister et al 2006; Rottiers and Antebi 2006), the best studied. Among the insects, a gene similar to the Drosophila methoprene tolerant protein gene (Met), possibly a juvenile hormone– (JH) dependent transcription factor, is upregulated early in Culex pipiens L diapause (Robich et al 2007), an unexpected observation because this species enters diapause in the absence of JH. mRNA for an ETS transcription factor homologue is enriched during Bombyx mori embryonic diapause (Suzuki et al 1999), and a POU factor may affect transcription of the B. mori diapause hormone (DH) and pheromone biosynthesis-activating neuropeptide (PBAN) gene (Zhang et al 2004a). B. mori DH influences the ovaries of pharate adults, producing diapause eggs in the following generation. Regulation of the DH-PBAN gene in Helicoverpa armigera, an insect with pupal diapause, involves multiple cis-elements and DNA binding proteins, possibly including a basic helix-loop-helix transcription factor named Har-DHMBP-3 (Hong et al 2006). H. armigera DH, in contrast to the situation in B. mori, is thought to promote growth rather than diapause (Xu and Denlinger 2003; Zhang et al 2004b), perhaps reflecting mechanistic differences between embryonic and pupal diapauses. In the final insect case 2 B. mori transcription factors may function in diapause termination (Moribe et al 2001; Shiomi et al 2005). Analysis by differential gene expression tentatively identified transcriptional regulatory components during delayed implantation (Hamatani et al 2004; Lopes et al 2004). However, in spite of extensive effort, definitive identification of transcription factors promoting diapause is accomplished only for C. elegans larvae that, unlike most invertebrates, undergo diapause upon exposure to adverse environmental conditions rather than in their anticipation.
Identification of cell processes regulated by p8 is underway but conclusions remain tenuous because results are contradictory, genetic redundancy is unexplored for the protein, and its function probably is influenced by interaction with different partners. For example, p8 may buttress defense against cell injury (Vasseur et al 2004; Malicet et al 2006b) and promote growth (Vasseur et al 1999a; Garcia-Montero et al 2001; Päth, et al 2006; Malicet et al 2006b). In contrast, p8 also inhibits cell growth and suppresses tumors (Bratland et al 2000; Vasseur et al 2002a, 2002b; Zinke et al 2002; Malicet et al 2003; Jiang et al 2005, 2006) while maintaining tumor phenotype by a mechanism perhaps different from growth promotion per se (Ree et al 1999; Su et al 2001; Vasseur et al 2002b; Mohammad et al 2004). p8 is both proapoptotic (Vasseur et al 2002a; Carracedo et al 2006; Plant et al 2006) and antiapoptotic (Su et al 2001; Giroux et al 2006), with inhibition thought to depend on interaction with prothymosin α (Malicet et al 2006a). p8 may stop growth when cells of Drosophila larvae experience nutrient deprivation (Zinke et al 2002), as occurs during diapause. The multiplicity of p8 activities is likely to depend on binding partner diversity (Hoffmeister et al 2002; Malicet et al 2003; Jiang et al 2005), with variation from 1 cell type to another and upon exposure to dissimilar environments. That p8 affects cell growth and apoptosis is especially pertinent, demonstrating this transcription cofactor has the capacity to regulate these key processes during Artemia embryo diapause.
To summarize, the gene for p8, a stress-inducible transfection cofactor with the ability to either promote or inhibit both cell growth and apoptosis, is synthesized specifically in diapause-destined Artemia embryos. Artemia p8 mRNA synthesis crests at day 1 postfertilization, prior to undergoing precipitous decline. The p8 protein peaks a day later and persists at about the same level throughout cyst development. As such, and because it resides in Artemia nuclei, p8 is an excellent candidate to exert transcriptional regulation during diapause. Pertinent activities include promotion of genes indigenous to the diapause development pathway, including those inhibiting apoptosis, silencing genes that promote cell growth, and differential expression of metabolic enzyme genes. In this context, the 5′-regulatory sequence of the p26 gene, the only other diapause upregulated gene in Artemia for which this region is available, contains a basic helix-loop-helix transcription factor binding site at -185-cagctg-180 (Qiu et al 2006; Hong et al 2006). Experiments are underway to determine p8 effects on the expression of p26 and other genes upregulated in diapause-destined Artemia embryos.
Acknowledgments
This work was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (T.H.M.).
REFERENCES
- Anderson E, Lochhead JH, Lochhead MS, Huebner E. The origin and structure of the tertiary envelope in thick-shelled eggs of the brine shrimp, Artemia. J Ultrastruct Res. 1970;32:497–525. doi: 10.1016/s0022-5320(70)80025-9. [DOI] [PubMed] [Google Scholar]
- Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol. 2006;190:191–202. doi: 10.1677/joe.1.06856.0022-0795(2006)190[0191:ESICEC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bratland Å, Risberg K, and Maelandsmo GM. et al. 2000 Expression of a novel factor, com1, is regulated by 1,25-dihydroxyvitamin D3 in breast cancer cells. Cancer Res. 60:5578–5583. [PubMed] [Google Scholar]
- Carracedo A, Lorente M, and Egia A. et al. 2006 The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 9:301–312. [DOI] [PubMed] [Google Scholar]
- Cartharius K, Frech K, and Grote K. et al. 2005 MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 21:2933–2942. [DOI] [PubMed] [Google Scholar]
- Chen T, Villeneuve TS, Garant KA, Amons R, MacRae TH. Functional characterization of artemin, a ferritin homologue synthesized in Artemia embryos during encystment and diapause. FEBS J. 2007;274:1093–1101. doi: 10.1111/j.1742-4658.2007.05659.x.1742-464X(2007)274[1093:FCOAAF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Clegg JS. Embryos of Artemia franciscana survive four years of continuous anoxia: the case for complete metabolic rate depression. J Exp Biol. 1997;200:467–475. doi: 10.1242/jeb.200.3.467.0022-0949(1997)200[0467:EOAFSF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Clegg JS, Jackson SA. The metabolic status of quiescent and diapause embryos of Artemia franciscana (Kellogg) ArchHydrobiol Spec Issue Advanc Limnol. 1998;52:425–439. [Google Scholar]
- Clegg JS, Jackson SA, Popov VI. Long-term anoxia in encysted embryos of the crustacean, Artemia franciscana: viability, ultrastructure, and stress proteins. Cell Tissue Res. 2000;301:433–446. doi: 10.1007/s004410000249.1432-0878(2000)301[0433:LAIEEO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Denlinger DL. Regulation of diapause. Annu Rev Entomol. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137.0066-4170(2002)047[0093:ROD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Encinar JA, Mallo GV, and Mizyrycki C. et al. 2001 Human p8 is a HMG-I/Y-like protein with DNA binding activity enhanced by phosphorylation. J Biol Chem. 276:2742–2751. [DOI] [PubMed] [Google Scholar]
- García-Montero AC, Vasseur S, Giono LE, Canepa E, Monero S, Dagorn JC, Iovanna JL. Transforming growth factor β-1 enhances Smad transcriptional activity through activation of p8 gene expression. Biochem J. 2001;357:249–253. doi: 10.1042/0264-6021:3570249.0264-6021(2001)357[0249:TGFEST]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux V, Malicet C, Barthet M, Gironella M, Archange C, Dagorn J-C, Vasseur S, Iovanna JL. p8 is a new target of gemcitabine in pancreatic cancer cells. Clin Cancer Res. 2006;12:235–241. doi: 10.1158/1078-0432.CCR-05-1700.1078-0432(2006)012[0235:PIANTO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Goruppi S, Kyriakis JM. The pro-hypertropic basic helix-loop-helix protein p8 is degraded by the ubiquitin/proteasome system in a protein kinase B/Akt- and glycogen synthase kinase-3-dependent manner, whereas endothelin induction of p8 mRNA and renal mesangial cell hypertrophy require NFAT4. J Biol Chem. 2004;279:20950–20958. doi: 10.1074/jbc.M312401200.0021-9258(2004)279[20950:TPBHPP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hamatani T, Daikoku T, Wang H, Matsumoto H, Carter MG, Ko MSH, Dey SK. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc Natl Acad Sci U S A. 2004;101:10326–10331. doi: 10.1073/pnas.0402597101.1091-6490(2004)101[10326:GGEAIM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister A, Ropolo A, and Vasseur S. et al. 2002 The HMG-I/Y-related protein p8 binds to p300 and Pax2 trans-activation domain-interacting protein to regulate the trans-activation activity of the Pax2A and Pax2B transcription factors on the glucagon gene promoter. J Biol Chem. 277:22314–22319. [DOI] [PubMed] [Google Scholar]
- Hong B, Zhang Z-F, Tang S-M, Yi Y-Z, Zhang T-Y, Xu W-H. Protein-DNA interactions in the promoter region of the gene encoding diapause hormone and pheromone biosynthesis activating neuropeptide of the cotton bollworm, Helicoverpa armigera. Biochim Biophys Acta. 2006;1759:177–185. doi: 10.1016/j.bbaexp.2006.03.003.0006-3002(2006)1759[0177:PIITPR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Igarashi T, Kuroda H, Takahashi S, Asashima M. Cloning and characterization of the Xenopus laevis p8 gene. Develop Growth Differ. 2001;43:693–698. doi: 10.1046/j.1440-169x.2001.00613.x.1440-169X(2001)043[0693:CACOTX]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jiang WG, Davies G, Fodstad O. Con-1/P8 in oestrogen regulated growth of breast cancer cells, the ER-β connection. Biochem Biophys Res Commun. 2005;330:253–262. doi: 10.1016/j.bbrc.2005.02.157.0006-291X(2005)330[0253:PIORGO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jiang WG, Davies G, Martin TA, Kynaston H, Mason MD, Fodstad O. Com-1/p8 acts as a putative tumour suppressor in prostate cancer. Int J Mol Med. 2006;18:981–986.1107-3756(2006)018[0981:PAAAPT]2.0.CO;2 [PubMed] [Google Scholar]
- Jones S 2004 An overview of the basic helix-loop-helix proteins. Genome Biol. 5:226. 1–226.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koštál V. Eco-physiological phases of insect diapause. J Insect Physiol. 2006;52:113–127. doi: 10.1016/j.jinsphys.2005.09.008.0022-1910(2006)052[0113:EPOID]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Langdon CM, Bagshaw JC, MacRae TH. Tubulin isoforms in the brine shrimp, Artemia: primary gene products and their posttranslational modification. Eur J Cell Biol. 1990;52:17–26.0171-9335(1990)052[0017:TIITBS]2.0.CO;2 [PubMed] [Google Scholar]
- Liang P, Amons R, Clegg JS, MacRae TH. Molecular characterization of a small heat shock/α-crystallin protein in encysted Artemia embryos. J Biol Chem. 1997;272:19051–19058. doi: 10.1074/jbc.272.30.19051.0021-9258(1997)272[19051:MCOASH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liang P, MacRae TH. The synthesis of a small heat shock/α-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev Biol. 1999;207:445–456. doi: 10.1006/dbio.1998.9138.1095-564X(1999)207[0445:TSOASH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lopes FL, Desmarais JA, Murphy BD. Embryonic diapause and its regulation. Reproduction. 2004;128:669–678. doi: 10.1530/rep.1.00444.0034-4958(2004)128[0669:EDAIR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- MacRae TH. Molecular chaperones, stress resistance, and development in Artemia franciscana. Semin Cell Dev Biol. 2003;14:251–258. doi: 10.1016/j.semcdb.2003.09.019.1084-9521(2003)014[0251:MCSRAD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- MacRae TH. Diapause: diverse states of developmental and metabolic arrest. J Biol Res. 2005;3:3–14. [Google Scholar]
- Malicet C, Giroux V, Vasseur S, Dagorn JC, Neira JL, Iovanna JL. Regulation of apoptosis by the p8/prothymosin α complex. Proc Natl Acad Sci U S A. 2006a;103:2671–2676. doi: 10.1073/pnas.0508955103.1091-6490(2006)103[2671:ROABTP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicet C, Hoffmeister A, Moreno S, Closa D, Dagorn J-C, Vasseur S, Iovanna JL. Interaction of the stress protein p8 with Jab1 is required for Jab1-dependent p27 nuclear-to-cytoplasm translocation. Biochem Biophys Res Commun. 2006b;339:284–289. doi: 10.1016/j.bbrc.2005.11.018.0006-291X(2006)339[0284:IOTSPP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Malicet C, Lesavre N, Vasseur S, and Iovanna JL 2003 p8 inhibits the growth of human pancreatic cancer cells and its expression is induced through pathways involved in growth inhibition and repressed by factors promoting cell growth. Mol Cancer. 2:37. http://www.molecular-cancer.com/content/2/1/37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo GV, Fiedler F, Calvo EL, Ortiz EM, Vasseur S, Keim V, Morisset J, Iovanna JL. Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J Biol Chem. 1997;272:32360–32369. doi: 10.1074/jbc.272.51.32360.0021-9258(1997)272[32360:CAEOTR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eukaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000.1098-5549(2000)020[0429:HPROTI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad HP, Seachrist DD, Quirk CC, Nilson JH. Reexpression of p8 contributes to tumorigenic properties of pituitary cells and appears in a subset of prolactinomas in transgenic mice that hypersecrete luteinizing hormone. Mol Endocrinol. 2004;18:2583–2593. doi: 10.1210/me.2004-0163.0888-8809(2004)018[2583:ROPCTT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Moribe Y, Niimi T, Yamashita O, Yaginuma T. Samui, a novel cold-inducible gene, encoding a protein with a BAG domain similar to silencer of death domains (SODD/BAG-4), isolated from Bombyx diapause eggs. Eur J Biochem. 2001;268:3432–3442. doi: 10.1046/j.1432-1327.2001.02244.x.0014-2956(2001)268[3432:SANCGE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Päth G, Opel A, and Gehlen M. et al. 2006 Glucose-dependent expansion of pancreatic β-cells by the protein p8 in vitro and in vivo. Am J Physiol Endocrinol Metab. 291:E1168–E1176. [DOI] [PubMed] [Google Scholar]
- Plant SR, Wang Y, and Vasseur S. et al. 2006 Upregulation of the stress-associated gene p8 in mouse models of demyelination and in multiple sclerosis tissues. Glia. 53:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Bossier P, Wang X, Bojikova-Fournier S, MacRae TH. Diversity, structure, and expression of the gene for p26, a small heat shock protein from Artemia. Genomics. 2006;88:230–240. doi: 10.1016/j.ygeno.2006.02.008.0888-7543(2006)088[0230:DSAEOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Quirk CC, Seachrist DD, Nilson JH. Embryonic expression of the luteinizing hormone β gene appears to be coupled to the transient appearance of p8, a high mobility group-related transcription factor. J Biol Chem. 2003;278:1680–1685. doi: 10.1074/jbc.M209906200.0021-9258(2003)278[1680:EEOTLH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ree AH, Tvermyr M, and Engebraaten O. et al. 1999 Expression of a novel factor in human breast cancer cells with metastatic potential. Cancer Res. 59:4675–4680. [PubMed] [Google Scholar]
- Robich RM, Rinehart JP, Kitchen LJ, Denlinger DL. Diapause-specific gene expression in the northern house mosquito, Culex pipiens L., identified by suppressive subtractive hybridization. J Insect Physiol. 2007;53:235–245. doi: 10.1016/j.jinsphys.2006.08.008.0022-1910(2007)053[0235:DGEITN]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottiers V, Antebi A. Control of Caenorhabditis elegans life history by nuclear receptor signal transduction. Exp Gerontol. 2006;41:904–909. doi: 10.1016/j.exger.2006.06.062.0531-5565(2006)041[0904:COCELH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shiomi K, Fujiwara Y, and Atsumi T. et al. 2005 Myocyte enhancer factor 2 (MEF2) is a key modulator of the expression of the prothoracicotropic hormone gene in the silkworm, Bombyx mori. FEBS J. 272:3853–3862. [DOI] [PubMed] [Google Scholar]
- Su S-B, Motoo Y, and Iovanna JL. et al. 2001 Overexpression of p8 is inversely correlated with apoptosis in pancreatic cancer. Clin Cancer Res. 7:1320–1324. [PubMed] [Google Scholar]
- Sun Y, Bojikova-Fournier S, MacRae TH. Structural and functional roles for β-strand 7 in the α-crystallin domain of p26, a polydisperse small heat shock protein from Artemia franciscana. FEBS J. 2006;273:1020–1034. doi: 10.1111/j.1742-4658.2006.05129.x.1742-464X(2006)273[1020:SAFRFS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. Characterization of novel sequence motifs within N- and C-terminal extensions of p26, a small heat shock protein from Artemia franciscana. FEBS J. 2005;272:5230–5243. doi: 10.1111/j.1742-4658.2005.04920.x.1742-464X(2005)272[5230:CONSMW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sun Y, Mansour M, Crack JA, Gass GL, MacRae TH. Oligomerization, chaperone activity, and nuclear localization of p26, a small heat shock protein from Artemia franciscana. J Biol Chem. 2004;279:39999–40006. doi: 10.1074/jbc.M406999200.0021-9258(2004)279[39999:OCAANL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Suzuki MG, Terada T, Kobayashi M, Shimadu T. Diapause-associated transcription of BmEts, a gene encoding an ETS transcription factor homolog in Bombyx mori. Insect Biochem Mol Biol. 1999;29:339–347. doi: 10.1016/s0965-1748(99)00008-9.0965-1748(1999)029[0339:DTOBAG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Valacco MP, Varone C, Malicet C, Cánepa E, Iovanna JL, Moreno S. Cell growth–dependent subcellular localization of p8. J Cell Biochem. 2006;97:1066–1079. doi: 10.1002/jcb.20682.0730-2312(2006)097[1066:CGSLOP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vasseur S, Folch-Puy E, and Hlouschek V. et al. 2004 p8 improves pancreatic response to acute pancreatitis by enhancing the expression of the anti-inflammatory protein pancreatitis-associated protein I. J Biol Chem. 279:7199–7207. [DOI] [PubMed] [Google Scholar]
- Vasseur S, Hoffmeister A, Garcia-Montero A, Mallo GV, Feil R, Kühbandner S, Dagorn J-C, Iovanna JL. p8-deficient fibroblasts grow more rapidly and are more resistant to adriamycin-induced apoptosis. Oncogene. 2002a;21:1685–1694. doi: 10.1038/sj.onc.1205222.0950-9232(2002)021[1685:PFGMRA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vasseur S, Hoffmeister A, Garcia S, Bagnis C, Dagorn J-C, Iovanna JL. p8 is critical for tumor development induced by rasV12 mutated protein and E1A oncogene. EMBO Reports. 2002b;3:165–170. doi: 10.1093/embo-reports/kvf023.1469-221X(2002)003[0165:PICFTD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasseur S, Mallo GV, Fiedler F, Bödeker H, Cánepa E, Moreno S, Iovanna JL. Cloning and expression of the human p8, a nuclear protein with mitogenic activity. Eur J Biochem. 1999a;259:670–675. doi: 10.1046/j.1432-1327.1999.00092.x.0014-2956(1999)259[0670:CAEOTH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vasseur S, Mallo GV, Garcia-Montero A, Ortiz EM, Fiedler F, Cánepa E, Moreno S, Iovanna JL. Structural and functional characterization of the mouse p8 gene: promotion of transcription by the CAAT-enhancer binding protein α (C/EBPα) and C/ EBPβ trans-acting factors involves a C/EBP cis-acting element and other regions of the promoter. Biochem J. 1999b;343:377–383.0264-6021(1999)343[0377:SAFCOT]2.0.CO;2 [PMC free article] [PubMed] [Google Scholar]
- Villeneuve TS, Ma X, Sun Y, Oulton MM, Oliver AE, MacRae TH. Inhibition of apoptosis by p26: implications for small heat shock protein function during Artemia development. Cell Stress Chaperones. 2006;11:71–80. doi: 10.1379/CSC-154R.1.1466-1268(2006)011[0071:IOABPI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner RI, Clegg JS. Influence of trehalose on the molecular chaperone activity of p26, a small heat shock/α-crystallin protein. Cell Stress Chaperones. 2001;6:126–135. doi: 10.1379/1466-1268(2001)006<0126:iototm>2.0.co;2.1466-1268(2001)006[0126:IOTOTM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W-H, Denlinger DL. Molecular characterization of prothoracicotropic hormone and diapause hormone in Heliothis virescens during diapause, and a new role for diapause hormone. Insect Mol Biol. 2003;12:509–516. doi: 10.1046/j.1365-2583.2003.00437.x.0962-1075(2003)012[0509:MCOPHA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhang T-Y, Kang L, Zhang Z-F, Xu W-H. Identification of a POU factor involved in regulating the neuron-specific expression of the gene encoding diapause hormone and pheromone biosynthesis-activating neuropeptide in Bombyx mori. Biochem J. 2004a;380:255–263. doi: 10.1042/BJ20031482.0264-6021(2004)380[0255:IOAPFI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T-Y, Sun J-S, Zhang Q-R, Xu J, Jiang R-J, Xu W-H. The diapause hormone-pheromone biosynthesis activating neuropeptide gene of Helicoverpa armigera encodes multiple peptides that break, rather than induce, diapause. J Insect Physiol. 2004b;50:547–554. doi: 10.1016/j.jinsphys.2004.03.011.0022-1910(2004)050[0547:TDHBAN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zinke I, Schütz CS, Katzenberger JD, Bauer M, Pankratz MJ. Nutrient control of gene expression in Drosophila: microarray analysis of starvation and sugar-dependent response. EMBO J. 2002;21:6162–6173. doi: 10.1093/emboj/cdf600.1460-2075(2002)021[6162:NCOGEI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]