Abstract
The annual killifish Austrofundulus limnaeus inhabits ephemeral ponds in regions of northern South America, where they survive the periodic drying of their habitat as diapausing embryos. These diapausing embryos are highly resistant to a number of environmental insults such as high temperature, dehydration, anoxia, and increased salinity. Molecular chaperones are known to play a role in stabilizing protein structure and function during events of cellular stress. Relative levels of heat shock protein (Hsp)70 were measured in developing and diapausing embryos of A. limnaeus using quantitative Western blots. An inducible or embryo-specific form of Hsp70 is expressed during embryonic development in A. limnaeus and is elevated during diapause II in this species. Constitutive expression of Hsp70 during development may afford these embryos protection from environmental stresses during development more quickly than relying on the induction of a classic heat shock response.
INTRODUCTION
A variety of organisms can enter diapause, a state of developmental and metabolic dormancy, to avoid unfavorable environmental conditions. Embryonic diapause is a state of developmental arrest that precedes the onset of unfavorable conditions and typically occurs as part of the organism's natural developmental program (Hand 1991). Thus embryos enter diapause even under conditions that are favorable for development. Diapause, like other forms of metabolic dormancy such as hibernation and estivation, often is associated with an increase in resistance to environmental stress. The mechanisms that support increased and sometimes excessive resistance to environmental stress remain elusive (for a review see Jönsson 2003).
The annual killifish Austrofundulus limnaeus survives in ephemeral ponds by producing drought-tolerant diapausing embryos (Podrabsky et al 2001). The tolerance of these embryos to environmental perturbations such as anoxia, dehydration, and elevated salinity is orders of magnitude higher than other life history stages of A. limnaeus (Podrabsky et al 2001; Miller and Podrabsky, personal communication; Podrabsky et al 2007). Paradoxically, diapausing embryos of A. limnaeus have a severely depressed rate of protein synthesis (Podrabsky and Hand 1999), and thus are likely limited in their ability to respond to environmental stress through de novo gene expression. Thus, it is likely that embryos of A. limnaeus prepare for environmental stress that they will experience during diapause as part of their natural developmental program.
It appears that there is a suite of molecular changes that can protect an individual or tissue from environmentally induced stresses. This has been illustrated through a variety of studies, some utilizing genomic and proteomic methodologies, in species ranging from yeast to fish (Gasch 2002; Gasch and Werner-Washburne 2002; Kültz 2003, 2005; Gracey et al 2004; Buckley et al 2006). The environmental stress response has been characterized by gene expression analysis in yeast (Gasch 2002) and Kültz (2003) has identified a “minimal stress proteome” that appears to be conserved in nearly all forms of life. Molecular chaperones are known to be important in mediating the damaging effects of environmental challenges to proteins and other macromolecules in all domains of life and are almost invariably observed as part of the stress response (Feder and Hofmann 1999). Synthesis of stress-induced molecular chaperones, heat shock proteins (Hsps), is known to afford individuals resistance to subsequent stressful events of either the same insult (induced tolerance) or to different insults, a process termed crosstolerance.
In this paper we investigate the expression of 70 kDa class heat shock proteins during active development and diapause in embryos of A. limnaeus. An Hsp70 isoform is expressed constitutively during early development and diapause in A. limnaeus at levels consistent with heat shocked adults of A. limnaeus and other species of fish. This isoform of Hsp70 appears to be heat inducible in adult liver and in developing embryos. The production of this protein under nonstressful conditions may be indicative of a preparation for environmental stresses to be encountered during diapause, when the ability to produce heat shock proteins may be severely limited.
RESULTS AND DISCUSSION
Isolation and characterization of embryos
This is the first report of total protein and DNA content in yolk-free embryos of A. limnaeus. Separation of the embryonic cells and tissues from the yolk mass yields total protein and DNA contents that are highly reproducible. Total DNA content (Fig 1A) of the embryonic tissues increases from 33.7 ± 1.8 to 122 ± 2.7 ng/embryo (mean ± standard error of mean [SEM]) from 4 to 16 days postfertilization ([dpf]; Analysis of Variance (ANOVA), P ≪ 0.0001). After 16 dpf, total DNA content does not change significantly through 72 dpf, which indicates that cell proliferation ceases 8 d prior to entry into diapause II when morphological development is arrested. This pattern is consistent with previous observations of metabolism in A. limnaeus embryos and suggests that the changes in embryo morphology that are observed between 16 and 24 dpf are likely due to cell rearrangement and not the production of new cells. In 4 dpf embryos, about 21% of the total embryo (yolk + embryonic tissues) DNA (160 ± 10 ng/embryo) reported by Podrabsky and Hand (1999) is found in the embryonic cells. For embryos 12 dpf through diapause II, about 32% of the total embryo DNA (281 ± 10 ng/embryo) is found in the embryonic cells. It is well documented for several species of aquatic vertebrates that a large amount of DNA can be found in the yolk and is likely of mitochondrial origin (Dawid 1965, 1966; Shmerling 1965; Baltus et al 1968).
Fig 1.
Total protein and DNA content in isolated embryos of Austrofundulus limnaeus. (A) Total DNA and (B) total protein content increases during early development between 4 and 16 days postfertilization (dpf). Neither total DNA nor protein content changes after 16 dpf. The protein: DNA ratio (C) is highest during early development. Symbols that share a bar on the same level are not statistically different (Student-Neuman-Keul (SNK), P > 0.05). Symbols represent means ± standard error of mean, n = 3. Letters at the top of panel A refer to major developmental landmarks: E, completion of epiboly; R, reaggregation of blastomeres; A, formation of the embryonic axis; 3S, 3-somite embryo; H, beginning of embryonic heart beat; 16S, 16-somite embryo; 28S, 28-somite embryo; ED, early diapause II; SD, steady-state diapause II. Adult fish and embryos were cared for using established laboratory protocols (Podrabsky 1999). Embryos were washed in several washes of N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid (HEPES) buffered Yamamoto's solution (128 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 20 mM HEPES, pH = 7.2) and then placed in a Petri dish and separated from the yolk with a pair of fine forceps. The isolated embryonic tissues then were transferred and washed twice with gentle agitation in buffered Yamamoto's solution as above. The washed isolated embryo tissues then were placed directly into 100 μL of lysis buffer (2% sodium dodecylsulfate, 1 mM ethylenediamine-tetraacetic acid [EDTA], 0.25 mg/mL Pefabloc, 10 μg/mL Pepstatin, and 10 μg/mL leupeptin, 32 mM Tris-HCl, pH = 6.8) in a 1.5-mL microcentrifuge tube. The samples were frozen at −80°C to aid in cellular disruption and then incubated in a heat block at 100°C for 2.5 min, briefly homogenized in the microcentrifuge tube with a Teflon pellet pestle, and returned to the heat block for 5 min. The sample then was subjected to 14 000 × g for 15 min to pellet the insoluble fraction and the supernatant retained and stored at −20°C. The whole process from puncturing the embryo to immersion in the lysis buffer took no longer than 1 min for each embryo. Total protein content was determined for each sample using the MicroBCA™ method according to the manufacturer's instructions (Pierce). Total DNA content was determined using a fluorescence assay employing Hoechst dye as outlined in Teare et al (1997)
Total protein (Fig 1B) increases during early development from 1.7 ± 0.2 to 5.5 ± 0.2 μg/embryo from 4 to 16 dpf (ANOVA, P = 0.0002). Thus, embryonic tissues account for only about 1% at 4 dpf and 6% at 20 dpf of the total protein reported by Podrabsky and Hand (1999). Between 16 and 72 dpf there is a slight decrease in protein content (mean decrease of 1.6 μg/embryo). Even though this decrease is not statistically significant, it may be functionally important because recent evidence (Podrabsky et al, personal communication) suggests that the free amino acid content of whole embryos increases during diapause, and these amino acids may arise from protein degradation.
The protein to DNA ratio (Fig 1C) peaks at 8 dpf and then declines steadily as embryos develop towards and enter diapause II (ANOVA, P = 0.005). Embryos at 8 dpf are characterized by extensive cell migrations associated with dispersion and reaggregation of the embryonic blastomeres (Wourms 1972). This increased level of cellular protein compared to DNA content partly contributes to the apparently lower levels of Hsp70 protein in these cells when expressed per unit protein (see Levels of Hsp70 section). The underlying causes for this pattern are unknown.
Levels of Hsp70
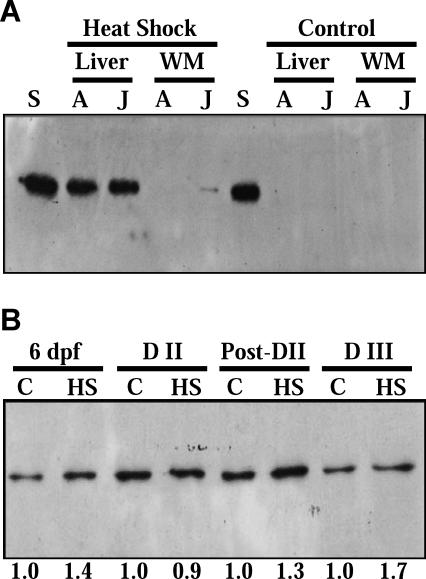
The antibody used in this study crossreacts with a constitutively expressed Hsp (bovine Hsc70, the normalization standard) and an inducible Hsp70 in the liver tissue of adult and juvenile A. limnaeus, as well as in embryos of A. limnaeus (Fig 2A,B). However, this antibody does not crossreact with heat shock cognate (Hsc) 70 in either the white muscle or liver of adult or juvenile A. limnaeus.
Fig 2.
Representative data indicating that the heat shock protein (Hsp) 70 antibody used in this experiment recognizes an inducible heat shock protein in Austrofundulus limnaeus. (A) The antibody does not react with any proteins in adult liver and white muscle (WM) from control (nonstressed) fish. However, the antibody does recognize an inducible form in the liver of heat-stressed adults, and weakly in the WM of heat-stressed juveniles. S, heat shock cognate (Hsc) 70 standard lane loaded with 100 ng of purified bovine Hsc70; A, adult; J, juvenile. (B) The antibody recognizes an Hsp70 protein in embryos of A. limnaeus that appears to be of the same molecular weight as an inducible Hsp70. C, control embryos; HS, heat shocked; 6 dpf, embryos at 6 days postfertilization (prediapause II); D II, diapause II; post–D II, 4 days postdiapause II; D III, diapause III. The numbers under the photograph indicate the induction of Hsp70 in those bands relative to the control within each developmental stage. Each lane was loaded with 6 μg of total protein. Embryos (groups of 20) were exposed to a 35°C heat shock for 1 h in a 20-mL glass vial containing 15 mL of fully oxygenated embryo medium. The suspensions were gently mixed every 10–15 min to ensure that the embryos were exposed to aerobic conditions. Embryos were allowed to recover from the heat shock for 1 h at 25°C prior to the isolation of the embryonic tissues. Control embryos were kept at 25°C, but otherwise treated exactly as the embryos subjected to heat shock. Western blots were performed using standard protocols (Tomanek and Somero 2002; Buckley and Hofmann 2004). Extracted proteins were electrophoretically separated and transferred to a nitrocellulose membrane. Membranes were blocked in Tris-buffered saline ([TBS], 137 mM NaCl, 10 mM Tris-HCl, pH = 7.4) containing 5% Carnation nonfat dried milk (Nestle) for 1 h with gentle agitation. The membranes were then washed once in TBST (TBS with 0.1% Tween 20 w/v) for 5 min, and twice in TBS for 5 min each. This washing procedure was used after each antibody incubation. The membranes were incubated in primary antibody for 1 h (MA3-001, Affinity Bioreagents, 1:1000 dilution), secondary bridging antibody (AI-4000, Vector Laboratories, 1:5000 dilution) for 30 min, and protein A:HRP (1:5000 dilution, BioRad) for 30 min. Immunoprobes were detected by exposing ECL™ hyperfilm to membranes soaked in ECL™ reagent (Amersham) according to the manufacturer's instructions. Relative amounts of Hsp70 protein were estimated using densitometry on digital images of the films. Each blot contained 1 lane loaded with 100 ng of purified bovine Hsc70 (SPP750, Stressgen) for standardization between gels. Protein amounts are reported relative to the 100 ng standard on each gel
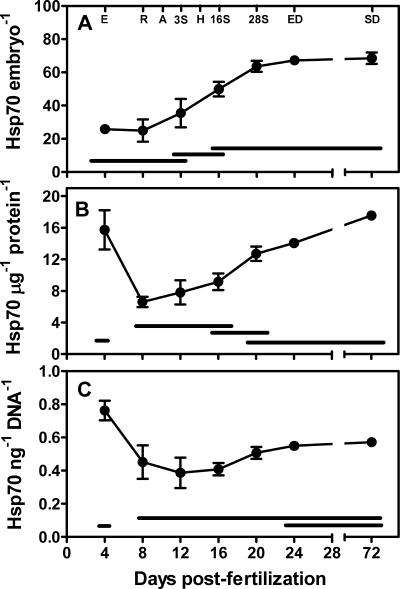
A 70 kDa–class heat shock protein is expressed during early development through diapause II in embryos of A. limnaeus (Fig 3) and throughout embryonic development in this species (Fig 2B). The exact identity of this isoform remains uncertain, but it either represents an Hsc70 that is highly expressed during development but not in adults, or an inducible Hsp70 that is expressed constitutively during development. The levels of Hsp70/μg protein expressed in early embryos of A. limnaeus are similar to the amount of Hsp70 observed in heat shocked tissues of the goby Gillichthys mirabilis quantified using the same methods employed in this study (B. Buckley, personal communication). When normalized on a per embryo basis, relative levels of this Hsp70 increase during early development, with the diapause II embryos containing the highest amounts (Fig 3A). However, if the Hsp70 content is expressed relative to total protein (Fig 3B) or total DNA content (Fig 3C), a much more complicated pattern is observed, with high amounts of Hsp70 early in development (4 dpf) and decreasing levels during subsequent development. Due to the role of Hsp70 as a molecular chaperone, the most relevant way to express the abundance and potential activity of this protein is likely as a function of the total cellular protein. When expressed in this manner, 4 dpf embryos and diapause II embryos have the highest amount of Hsp70, which likely translates to the highest amount of protection from environmental insults. The high relative expression of Hsp70 in 4 dpf embryos perhaps is counterintuitive when the biology of these embryos is considered. Early embryos tend to be more sensitive to environmental stresses such as desiccation (Podrabsky et al 2001), anoxia (Podrabsky et al 2007), and salinity (Miller and Podrabsky, personal communication) than are diapausing embryos. Perhaps the physiology of these embryos is more sensitive to perturbation, and thus higher levels of Hsps are required to protect the embryos.
Fig 3.
Relative amounts of heat shock protein (Hsp) 70 expressed (A) per embryo, (B) per μg protein, or (C) per ng DNA in isolated embryos of Austrofundulus limnaeus. Symbols that share a bar on the same level are not statistically different (SNK, P > 0.05). Symbols represent means ± standard error of mean, n = 3. Letters at the top of panel A indicate major developmental landmarks as defined in Figure 1. Embryos were incubated in embryo medium containing 10 mg/L gentamycin sulfate at 25°C in the dark (Podrabsky 1999). These conditions are considered nonstressful and have been illustrated previously to promote entry of embryos into diapause (Podrabsky and Hand 1999)
Diapausing embryos of A. limnaeus are known to have severely reduced levels of protein synthesis (Podrabsky and Hand 2000), and perhaps the elevated expression of Hsp70 during early development and diapause is present to protect the embryos during dormancy when their ability to respond to environmental stress via de novo protein synthesis presumably would be severely limited. Data presented in Figure 2 support the lack of a major induction of Hsp70 in heat shocked diapause II embryos, and there is clearly an induction of heat shock proteins in the actively developing embryos. Elevated levels of Hsp70 have been associated with survival of dehydration stress in tardigrades (Schill et al 2004) and with survival of anoxia in the brain of freshwater turtles (Lutz and Milton 2004). Both dehydration stress and anoxia typically are experienced by embryos of A. limnaeus in their natural environment, and thus it is logical to hypothesize that elevated levels of Hsp70 may help to mediate protein damage caused by these stresses during early development and diapause.
Only a handful of studies have examined the expression of 70 kDa heat shock proteins during teleost fish development, and it appears that inducible Hsp70 is not typically expressed during nonstressful conditions in teleost embryos (Heikkila et al 1986; Krone et al 1997; Lele et al 1997). One exception is the synthesis of Hsp70 in the developing eye of the zebrafish, Brachydanio rerio, which is known to be essential for normal eye development (Evans et al 2005). Expression of an inducible form of Hsp70 during the early development of A. limnaeus may be associated with the unique ability of these embryos to enter metabolic dormancy during diapause, a state that affords them a high tolerance to a number of environmental insults. This conclusion is supported by reports of elevated expression of Hsp70 during diapause in insects (Rinehart et al 2000; Teixeira and Polavarapu 2005).
Overexpression of Hsp70 in Drosophila has been shown to have negative effects on cellular and organismal growth and development (Feder et al 1992; Krebs and Feder 1997). If there are negative effects on developmental rate due to the elevated levels of Hsp70 during normal development in embryos of A. limnaeus, then this may help to explain why this species develops much more slowly than other Cyprinodont fish with similar sized eggs such as Fundulus heteroclitus. This species hatches after only 10–14 days when incubated at 20°C (DiMichelle and Taylor 1980), a time at which embryos of A. limnaeus are just starting to form an embryonic axis when incubated at 25°C (Podrabsky and Hand 1999). Rinehart et al (2000) discuss the implications of elevated Hsp70 during insect diapause and conclude that overexpression may have few deleterious effects in this situation due to the reduced cell division and growth associated with diapause. However, in their experimental system, Hsp70 expression is associated with entry into diapause, and not with active prediapause development, as is the case for A. limnaeus. Perhaps reduced rates of early development in A. limnaeus are a trade-off required to support increased ability to respond quickly, without a classic heat shock response, to environmental insults in their harsh and sometimes unpredictable environment.
Acknowledgments
We would like to thank B. Buckley for helpful comments on the manuscript and L. Tomanek for assistance with optimizing the western blot protocols. This work was supported by National Science Foundation grants IBN 0344578 (J.E.P.) and IBN 0133184 (G.N.S.)
REFERENCES
- Baltus E, Hanocq-Quertier J, Brachet J. Isolation of deoxyribonucleic acid from the yolk platelets of Xenpus laevis oocyte. Proc Natl Acad Sci U S A. 1968;61:469–476. doi: 10.1073/pnas.61.2.469.1091-6490(1968)061[0469:IODAFT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley BA, Gracey AY, Somero GN. The cellular response to heat stress in the goby Gillichthys mirabilis: a cDNA microarray and protein-level analysis. J Exp Biol. 2006;209:2660–2677. doi: 10.1242/jeb.02292.0022-0949(2006)209[2660:TCRTHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Buckley BA, Hofmann GE. Magnitude and duration of thermal stress determine kinetics of hsp gene regulation in the goby Gillichthys mirabilis. Physiol Biochem Zool. 2004;77:570–581. doi: 10.1086/420944.1522-2152(2004)077[0570:MADOTS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dawid IB. Deoxyribonucleic acid in amphibian eggs. J Mol Biol. 1965;12:581–599. doi: 10.1016/s0022-2836(65)80313-8.0022-2836(1965)012[0581:DAIAE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dawid IB. Evidence for the mitochondrial origin of frog egg cytoplasmic DNA. Proc Natl Acad Sci U S A. 1966;56:269–276. doi: 10.1073/pnas.56.1.269.1091-6490(1966)056[0269:EFTMOO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMichele L, Taylor MH. The environmental control of hatching in Fundulus heteroclitus. J Exp Zool. 1980;214:181–187.1097-010X(1980)214[0181:TECOHI]2.0.CO;2 [Google Scholar]
- Evans TG, Yamamoto Y, Jeffrey WR, Krone PH. Zebrafish Hsp70 is required for embryonic lens formation. Cell Stress Chap. 2005;10:66–78. doi: 10.1379/CSC-79R.1.1466-1268(2005)010[0066:ZHIRFE]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243.0066-4278(1999)061[0243:HSPMCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Feder JH, Rossi JM, Solomon J, Solomon N, Linquist S. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes Develop. 1992;6:1402–1413. doi: 10.1101/gad.6.8.1402.0890-9369(1992)006[1402:TCOEHI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gasch AP 2002 The Environmental Stress Response: a common yeast response to environmental stresses. In: Yeast Stress Responses, ed Hohmann S., Mager P. vol 1, Topics in Current Genetics, (ed Hohmann S) Springer-Verlag, Heidelberg, 11–70. [Google Scholar]
- Gasch AP, Werner-Washburne M. The genomics of yeast responses to environmental stress and starvation. Funct Integr Genomics. 2002;2:181–192. doi: 10.1007/s10142-002-0058-2.1438-7948(2002)002[0181:TGOYRT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gracey AY, Fraser EJ, Li W, Fang Y, Taylor RR, Rogers J, Brass A, Cossins AR. Coping with cold: an integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate. Proc Natl Acad Sci U S A. 2004;101:16970–16975. doi: 10.1073/pnas.0403627101.1091-6490(2004)101[16970:CWCAIM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand SC. Metabolic dormancy in aquatic invertebrates. Adv Comp Environ Physiol. 1991;8:1–50.0938-2763(1991)008[0001:MDIAI]2.0.CO;2 [Google Scholar]
- Heikkila JJ, Browder LW, Gedamu L, Nickells RW, Schultz GA. Heat shock gene expression in animal embryonic systems. Can J Genet Cytol. 1986;28:1093–1105. doi: 10.1139/g86-153.0008-4093(1986)028[1093:HSGEIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jönsson KI. Causes and consequences of excess resistance in cryptobiotic metazoans. Physiol Biochem Zool. 2003;76:429–435. doi: 10.1086/377743.1522-2152(2003)076[0429:CACOER]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Krebs RA, Feder ME. Deleterious consequences of Hsp70 overexpression in Drosophila melanogaster larvae. Cell Stress Chap. 1997;2:60–71. doi: 10.1379/1466-1268(1997)002<0060:dcohoi>2.3.co;2.1466-1268(1997)002[0060:DCOHOI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone PH, Sass JB, Lele Z. Heat shock protein gene expression during embryonic development of the zebrafish. CMLS Cell Mol Life Sci. 1997;53:122–129. doi: 10.1007/PL00000574.1420-682X(1997)053[0122:HSPGED]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kültz D. Evolution of the cellular stress proteome: from monophyletic origin to ubiquitous function. J Exp Biol. 2003;206:3119–3124. doi: 10.1242/jeb.00549.0022-0949(2003)206[3119:EOTCSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kültz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635.0066-4278(2005)067[0225:MAEBOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lele Z, Engel S, Krone PH. hsp47 and hsp70 gene expression is differentially regulated in a stress- and tissue-specific manner in zebrafish embryos. Devel Gen. 1997;21:123–133. doi: 10.1002/(SICI)1520-6408(1997)21:2<123::AID-DVG2>3.0.CO;2-9.1520-6408(1997)021[0123:HAHGEI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lutz PL, Milton SL. Negotiating brain anoxia survival in the turtle. J Exp Biol. 2004;207:3141–3147. doi: 10.1242/jeb.01056.0022-0949(2004)207[3141:NBASIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Podrabsky JE. Husbandry of the annual killifish Austrofundulus limnaeus with special emphasis on the collection and rearing of embryos. Env Biol Fish. 1999;54:421–431.0378-1909(1999)054[0421:HOTAKA]2.0.CO;2 [Google Scholar]
- Podrabsky JE, Carpenter JF, Hand SC. Survival of water stress in annual fish embryos: dehydration avoidance and egg envelope amyloid fibers. Am J Physiol. 2001;280:R123–R131. doi: 10.1152/ajpregu.2001.280.1.R123.0002-9513(2001)280[R123:SOWSIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Podrabsky JE, Hand SC. The bioenergetics of embryonic diapause in an annual killifish, Austrofundulus limnaeus. J Exp Biol. 1999;202:2567–2580. doi: 10.1242/jeb.202.19.2567.0022-0949(1999)202[2567:TBOEDI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Podrabsky JE, Hand SC. Depression of protein synthesis during diapause in embryos of the annual killifish Austrofundulus limnaeus. Physiol Biochem Zool. 2000;73:799–808. doi: 10.1086/318106.1522-2152(2000)073[0799:DOPSDD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Podrabsky JE, Lopez JP, Fan TW-M, Higashi R, and Somero GN 2007 Extreme anoxia tolerance in embryos of the annual killifish Austrofundulus limnaeus: insights from a metabolomics analysis. J Exp Biol (in press). [DOI] [PubMed] [Google Scholar]
- Rinehart JP, Yocum GD, Denlinger DL. Developmental upregulation of inducible hsp70 transcripts, but not the cognate form, during pupal diapause in the flesh fly, Sarcophaga crassipalpis. Insect Biochem Mol Biol. 2000;30:515–521. doi: 10.1016/s0965-1748(00)00021-7.0965-1748(2000)030[0515:DUOIHT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schill RO, Steinbrück GHB, Köhler H-R. Stress gene (hsp70) sequences and quantitative expression in Milnesium tardigradum (Tardigrada) during active and cryptobiotic stages. J Exp Biol. 2004;207:1607–1613. doi: 10.1242/jeb.00935.0022-0949(2004)207[1607:SGHSAQ]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shmerling ZhG. Isolation and properties of DNA of sturgeon oocytes. Biochemistry (Moscow) 1965;30:96–104.0006-2979(1965)030[0096:IAPODO]2.0.CO;2 [PubMed] [Google Scholar]
- Teare JM, Islam R, Flanagan R, Gallagher S, Davies MG, Grabau C. Measurement of nucleic acid concentrations using the DyNA Quant and the GeneQuant. Biotechniques. 1997;22:1170–117. doi: 10.2144/97226pf02.0736-6205(1997)022[1170:MONACU]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Teixeira LAF, Polavarapu S. Expression of heat shock protein 70 after heat stress during pupal diapause in Rhagoletis mendax (Diptera: Tephritidae) Ann Entomol Soc Am. 2005;98:966–972.0013-8746(2005)098[0966:EOHSPA]2.0.CO;2 [Google Scholar]
- Tomanek L, Somero GN. Interspecific- and acclimation-induced variation in levels of heat-shock proteins 70 (hsp70) and 90 (hsp90) and heat shock transcription factor-1 (HSF1) in congeneric marine snails (genus Tegula): implications for regulation of hsp gene expression. J Exp Biol. 2002;205:677–685. doi: 10.1242/jeb.205.5.677.0022-0949(2002)205[0677:IAAVIL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wourms JP. Developmental biology of annual fishes I. Stages in the normal development of Austrofundulus myersi Dahl. J Exp Zool. 1972;182:143–168. doi: 10.1002/jez.1401820202.1097-010X(1972)182[0143:DBOAFI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]