Abstract
Environmental injury has been associated with endoplasmic reticulum (ER) stress, a response characterized by activation of the unfolded protein response, proteasomal degradation of proteins, and induction of HSPA5, also known as GRP78 or BiP. Although HSPA5 has been implicated in the stress response to environmental injury in several cell types, its role in the glomerular ER stress response is unknown. In this study, we evaluated HSPA5 activation profiles in rat glomerular mesangial cells (rGMCs) challenged with heavy metals (HgCl2 or Pb2+ acetate) or polycyclic aromatic hydrocarbons (PAHs, ie, benzo(a)pyrene [BaP]). Challenge of rGMCs with 1 or 10 μM HgCl2 or Pb2+ acetate increased HSPA5 mRNA and protein levels. The induction response was sensitive to transcriptional and translational inhibition by actinomycin D (AD) and cyclohexamide, respectively. HSPA5 mRNA was induced by 3 μM BaP in an AD-sensitive manner, but this response was unaffected by the presence of heavy metals. A promoter construct containing sequences that mediate thapsigargin (TH) inducibility of the HSPA5 promoter was refractory to both heavy metals and BaP. The HSPA5 induction response in rGMCs is conserved because it was reproduced with fidelity in immunolocalization experiments of HSPA5 protein in M15 and HEK293 cells in embryonic lines of murine and human origin, respectively. Collectively, these findings identify HSPA5 in the stress response of rGMCs and implicate regulatory mechanisms that are distinct from those involved in TH inducibility.
INTRODUCTION
Endoplasmic reticulum (ER) stress results in accumulation of misfolded proteins and initiation of the unfolded protein response (UPR). UPR involves transcriptional induction of folding enzymes and ER chaperones, translational attenuation to prevent further ER protein loading, and ER-associated protein degradation. ER-associated molecular chaperones play an essential role in the etiology of numerous diseases, with a rapidly increasing role in clinical practice (Brooks 1997). HSPA5 (also known as glucose-regulated protein 78 or BiP) is involved in the UPR. HSPA5 is a member of the heat shock protein 70 (Hsp70) family of proteins, which function as molecular chaperones by binding transiently to proteins traversing through the ER and facilitating their folding, assembly, and transport (Liu et al 1998). HSPA5 has also been implicated in the cellular response to altered intracellular Ca2+ homeostasis. HSPA5 recognizes unfolded polypeptides, inhibits intra- and intermolecular aggregation, and promotes oligomerization and proper folding. During the ER stress response, HSPA5 seals ER membranes at the luminal end of the translocon and transports aberrant polypeptides across the ER membrane for proteosomal degradation (Gething 1999; Michalak et al 2002; Rao et al 2002).
In the ER, HSPA5 exists as an interconvertible monomer and oligomer that can be posttranslationally phosphorylated and ADP ribosylated with only monomers binding unfolded or unassembled proteins. Each HSPA5 monomer contains an N-terminal ATPase catalytic site and a C-terminal substrate binding domain. The ATP bound form is associated with low-affinity binding, whereas the ADP bound form exhibits high affinity and slow exchange. Mechanistically, HSPA5 binds misfolded proteins until all BiP-binding motifs have been removed by proper folding.
The adaptive response to chemical injury has been associated with rapid induction of HSPA5 in several cell types. HSPA5 is induced by a variety of stressors, including misfolded proteins, depletion of Ca2+ stores following inhibition of ER-specific Ca2+-ATPase by thapsigargin (TH) or release of Ca2+ with A23187, and disturbance of the ER to a trans-Golgi network by Brefeldin A (Little et al 1994). HSPA5 levels increase under a variety of stressful conditions by a complex interplay of cis-elements and protein factors that bind to these sites and regulate transcription. Sequence homology analysis of the grp78 promoter region as defined by Resendez et al (1988) and Roy et al (1996) has identified several putative xenobiotic-regulated sequences, including the antioxidant/electrophile response element (ARE/EpRE), cAMP response element (CRE), and TPA response element (TRE). One or more of these elements can participate in the regulation of HSPA5 by heavy metals and other chemical stressors.
The inorganic or organic forms, or both, of mercury and lead have been implicated in various forms of human illness, with kidney and brain ranking highest among critical target organs associated with morbidity. In the kidney, inorganic mercury is associated with increased creatinine excretion, proteinuria, hematuria, and degeneration of convoluted tubules (Magos et al 1984; Hirszel et al 1985; Henry et al 1988). Animal studies involving chronic exposure to lead (Pb2+) have demonstrated renal tubular damage, a response characterized by accumulation of intranuclear inclusion bodies and renal cancer after high-dose exposures (Goyer and Rhyne 1973). Exposure of rat glioma cells to Pb2+ acetate results in HSPA5 induction and Pb2+ sequestration (Qian et al 2005a), suggesting that HSPA5 acts as a component of the Pb2+ tolerance mechanism in these cells.
HSPA5 regulation was initially thought to be controlled at the mRNA level via an internal ribosomal entry site (IRES 1; Luo et al 2003). However, more recent work has shown that ER stress increases translation efficiency, resulting in protein induction regardless of elements in the 5′ and 3′ UTR of mRNA (Gulow et al 2002). Others have implicated translational mechanisms in the regulation of HSPA5 (Baumeister et al 2005; Mao et al 2006). To date, the patterns of HSPA5 expression in glomerular cells have not been examined. The hypothesis being tested here is that HSPA5 is involved in the stress response of rGMCs triggered by environmental stressors, such as HgCl2, Pb2+ acetate, or benzo(a)pyrene (BaP). Evidence is presented that chemical stress in rGMCs induces HSPA5 mRNA and protein and that this induction response involves transcriptional and posttranscriptional mechanisms that differ from the well-characterized TH-inducible response.
MATERIALS AND METHODS
Materials
RPMI 1640 and M199 were purchased from Gibco-BRL (Grand Island, NY, USA). Other chemicals were purchased from Sigma Chemical Co (St Louis, MO, USA).
Cell culture and chemical treatments
rGMCs, M15 mouse mesonephric cells, and HEK293 human embryonic kidney cells were cultured in Dulbecco modified Eagle medium (Mediatech Inc, Herndon, VA, USA) containing 10% fetal bovine serum (Atlanta Biological, Lawrenceville, GA, USA) and 1% antibiotic-antimycotic (Gibco). rGMCs were seeded at a density of 200 cells/mm2 on 6 well plates or Nunc Lab Tek II 2 well chamber slides (Nunc, Naperville, IL, USA) with 2 mL of full media. Triplicate wells per group or 1 slide were challenged with 1 μM actinomycin D (AD), 100 nM thapsigargin (TH) ± AD, 1 or 10 μM HgCl2 ± AD, 1 or 10 μM Pb2+ acetate ± AD, 3 or 30 μM BaP ± AD, or equivalent volumes of dimethyl sulfoxide or ethanol for 16 hours. Concentrations of 1 and 10 μM HgCl2 and Pb2+ acetate are within the range that induces ER stress in rat C6 glioma cells (Qian et al 1995, 1999, 2001, 2005a), and consistent with environmental concentrations of these metals (Florea and Busselberg, 2006). Likewise, BaP exposures at 3 μM span the range of estimated human exposures 0.171–1.64 μg/day (Busbee et al 1990; USEPA 1990; Bral and Ramos 1997). None of the treatment regimens tested, except for 30 μM BaP, affect cell viability (data not shown). Similar combinations were employed with the translational inhibitor cycloheximide (Cyc). Cells were lysed and proteins extracted in 1 step with Pierce MPER protein extraction reagent plus protease inhibitors (Pierce, Rockford, IL, USA).
Western blot analysis
Protein (30 g) was loaded in each lane, separated on 4–12% Bis Tris gels (Invitrogen, Carlsbad, CA, USA), run at 200 V for 1 hour, and transferred to a polyvinylidene difluoride (PVDF) membrane as recommended in the manufacturer's protocol. HSPA5 was detected with a goat polyclonal antibody (1:200; Santa Cruz Biochemicals, Santa Cruz, CA, USA). A bovine anti-goat horseradish peroxidase–conjugated secondary antibody was employed for detection (1:25 000; Santa Cruz Biochemicals) and visualized by chemiluminescence (Amersham Biosciences). Exposures were captured on Pierce film and documented with an HP700c Scanjet digital scanner at 600 dpi. Kodak 1D 3.5 software was used to measure net intensities. The intensities from at least 3 blots were normalized to a Ponceau-S–visualized nonspecific band as a loading control and normalized to vehicle control.
RNA extraction and analysis
Total RNA was extracted from the cells with TRIzol® reagent (Invitrogen) according to the manufacturer's specifications. Cells were scraped with 1 mL of Tri reagent, allowed to sit at room temperature for 5 minutes to dissociate nucleoprotein complexes, combined with 0.2 mL of chloroform, vortexed, and allowed to sit at room temperature for 2 minutes. After centrifugation at 12 000 × g (4°C) for 15 minutes, the upper aqueous layer was mixed with an equal volume of isopropanol and stored at −20°C overnight. This solution was then centrifuged for 15 minutes at 12 000 × g (4°C), and the pellet was subsequently washed with 70% ethanol, dried, and resuspended in 20 μL of RNase-free water. RNA concentration was determined spectrophotometrically at 260 nm.
Quantitative reverse transcription polymerase chain reaction of HSPA5
RNA (3.39 μg) was reverse transcribed to cDNA with superscript TM First-Strand Synthesis System (Invitrogen). Taq DNA polymerase (Promega, Madison, WI, USA) was used to amplify cDNA. Specific primers used to synthesize the HSPA5 product were (forward primer [F] 5′-AACCGCATCACACCGTCGTATG-3′ and reverse primer [R] 5′-TCCGCCAACCAGAACAATCTC-3′). The reverse transcription (RT) reaction was carried out at 94°C for 15 seconds, 60°C for 15 seconds, and 68°C for 1 minute. The curves for polymerase chain reaction (PCR) cycle numbers vs PCR products were measured, and PCR cycle number was defined from the linear phase of the curve. After completion of PCR cycles, the reaction was incubated at 72°C for 7 minutes. The PCR products were separated by agarose gel electrophoresis and excised from 1% agarose gels containing ethidium bromide. Band density was quantified with Scion Image NIH Software. HSPA5 mRNA intensity was normalized to 28S and18S. The data shown is representative of results seen in 3 separate experiments.
Transfection and chloramphenicol acetyltransferase analysis
The HSPA5–chloramphenicol acetyltransferase (CAT) construct, a kind gift from Dr Amy Lee (UCLA, Los Angeles, CA, USA) consisted of a 422-bp fragment of the rat promoter (−456 to −34) fused 5′ to the CAT gene (Chang et al 1987; Wooden et al 1991). The control and HSPA5 constructs were transiently transfected into rGMCs with LipofectAMINE PLUS™ (Invitrogen) according to manufacture's instructions. Briefly, 24 hours before transfection, rGMCs were plated onto 6-well Falcon plates at a density of 200 cells/mm2. DNA (1 μg) was mixed with LipofectAMINE reagent and incubated at room temperature for 15 minutes. DNA–LipofectAMINE-plus™ complexes were added to each well containing fresh medium and incubated at 37°C for 3 hours. After transfection, fresh media was added for an additional 24 hours. Cells were challenged with BaP, HgCl2, and Pb2+ acetate as defined in individual experiments, washed with PBS, and lysed in 100 μL of 0.25 M Tris Cl (pH 7.8). The cell lysates were collected and centrifuged for 3 minutes at 14 000 rpm, and the resulting extracts were heated at 60°C to inactivate endogenous CAT activity. Harvested protein (1 μg) was incubated for 37°C for 1 hour in the presence of [14C]chloramphenicol and acetyl-CoA. The reaction was halted by extraction with 700 μL of ethyl acetate. The organic phase was dried down, and the material was resuspended in 30 μL of ethyl acetate and separated by thin-layer chromatography in silica plates with a chloroform:methanol (9:1) solvent. Radioactivity was counted with the Instant Imager.
Promoter analysis
To identify evolutionarily conserved sequences in the HSPA5 promoter, DNA sequences consisting of 2.7 Kb upstream of the +1 position of the HSPA5 gene from mouse and rat were analyzed with DiAlign and MatInspector software to align sequences and identify common transcription factor binding sites, respectively (www.genomatrix.de). The HSPA5 gene (also known as 78-kDa glucose-regulated protein) promoter region DNA sequence from Mus musculus was obtained from clone RP23-446N16 on chromosome 2 (accession AL929106;GI:24940314; http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nucleotide&val=24940314). The HSPA5gene promoter region DNA sequence from Rattusnorvegicus (Norway rat) was obtained from the chromosome3 genomic contig (accession NW_047652; GI:62644995; http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nucleotide&qty=1&c_start=1&list_uids=NW_047652.2&uids=&dopt=gbwithparts&dispmax=5_sendto=&from=begin&to=end).
Immunocytochemical analysis
M15 and HEK293 cells were seeded at 225 cells/mm2 in Labtek II chamber slides (Nunc). Cells were fixed with methanol:acetone (1:1), incubated in HSPA5 (N-20) goat polyclonal antibody (Santa Cruz) and Alexa Flour™ rabbit anti-goat IgG, counterstained with 4′,6-diamidino-2-phenylindole, and mounted with Anti Fade™ (Molecular Probes, Eugene, OR, USA). With a Zeiss Axiovert 200 microscope and Axiovision image acquisition software, 10 images per chamber were taken per group at 40× and stored as ZVI files.
Image analysis
ZVI files were imported into Zeiss KS300 3.0 and analyzed for percent HSPA5 objects per nuclei. The change in HSPA5 area was calculated with the threshold values set with the signals in the no-treatment group for samples exposed to primary HSPA5 antibody or blocking serum only.
Statistical analysis
SPSS12Eval was used to perform analysis of variance and least squares difference tests to determine significance between groups (P < 0.05). Error bars represent standard errors of the mean.
RESULTS
Activation of HSPA5 in rGMCs by chemical stress
rGMCs were exposed to HgCl2 (1 or 10 μM), Pb2+ acetate (1 or 10 μM), or TH (100 nM) for 16 hours in the presence or absence of AD (10 μM), a transcriptional inhibitor. Thapsigargin increased HSPA5 mRNA levels 2-fold, compared with controls (Fig 1). The activation response to TH was completely inhibited by AD, indicating that regulation of HSPA5 mRNA expression in rGMCs is mediated at the transcriptional level. Treatment of cells with HgCl2 and Pb2+ acetate modestly increased mRNA levels compared with control, but only cells treated with Pb2+ acetate exhibited AD sensitivity (Fig 1). Western blot analysis was completed next to determine whether chemical stress by heavy metals increases HSPA5 protein levels. Exposure to 1 or 10 μM HgCl2 and Pb2+ acetate induced HSPA5 protein, but induction was modest when compared with TH (Fig 2). The modulation of HSPA5 protein by 1 μM HgCl2 was not sensitive to AD, whereas the cellular response to 10 μM HgCl2 exhibited some sensitivity (Fig 2). Pb2+ acetate modestly induced HSPA5 protein, with AD sensitivity seen at the 10 μM concentration. The induction of HSPA5 protein by TH was inhibited by AD. With the exception of 1 μM Pb2+ acetate, Cyc cotreatment decreased HSPA5 protein levels in all treatment groups.
Fig 1.
HSPA5 mRNA profiles in metal-stressed rat glomerular mesangial cells (rGMCs). Quantitative reverse transcriptase–polymerase chain reaction measurements of HSPA5 mRNA were conducted in rGMCs exposed to 10 μM actinomycin D (AD), 100 nM thapsigargin (TH) ± AD, 1 and 10 μM HgCl2 ± AD, and 1 and 10 μM Pb2+ acetate ± AD. AD was used as a transcriptional inhibitor. Thapsigargin was used as a positive control for endoplasmic reticulum stress (Baumeister et al 2005) and HSPA5 induction. Total RNA was extracted and processed as described in Materials and Methods. Similar results were seen in 3 separate experiments
Fig 2.
HSPA5 protein expression profiles in metal-stressed rat glomerular mesangial cells (rGMCs). (A) Western blot analysis of HSPA5 protein in rGMCs exposed to 10 μM actinomycin (AD), 1 μM HgCl2 ± 10 μM AD or 5 μM cycloheximide (Cyc), 300 nM thapsigargin (TH) ± AD or Cyc. (B) Western blot analysis of HSPA5 protein in rGMCs exposed to 10 μM AD, 10 μM HgCl2 ± 10 μM AD or 5 μM Cyc. (C) Western blot analysis of HSPA5 protein in rGMCs exposed to dimethyl sulfoxide, 1 or 10 μM Pb2+ acetate ± 10 μM AD or 5 μM Cyc. Total protein was extracted and processed as described in Materials and Methods. Values represent data from 4 separate experiments, each normalized to Ponceau-S staining and no treatment
rGMC HSPA5 mRNA induction is promoter dependent
The HSPA5 promoter contains ARE/EpRE-, CCAAT-, CRE-, and TRE-like promoter elements (Fig 3). These elements are recognized xenobiotic-regulated sequences in genomic DNA (Dhakshinamoorthy et al 2000). To evaluate transcriptional inducibility profiles by chemical stress, rGMCs were transfected with the HSPA5-CAT construct upstream of the CAT gene (Chang et al 1987; Wooden et al 1991). rGMCs were exposed to 10 μM HgCl2 or Pb2+ acetate or to 100 nM TH and assayed for CAT reporter activity. Only TH significantly induced reporter activity. This finding was unexpected given that induction of HSPA5 mRNA and protein by heavy metals exhibited AD sensitivity.
Fig 3.
Transcriptional activation profiles in metal-stressed rat glomerular mesangial cells (rGMCs). HSPA5–chloramphenicol acetyltransferase (CAT) constructs were transiently transfected into rGMCs, and CAT activity was measured as described in Materials and Methods. CAT activities were measured in cells challenged with dimethyl sulfoxide, 100 nM TH, 10 μM HgCl2, or 10 μM Pb2+ acetate. Similar results were seen in 3 separate experiments with at least 3 replicate cultures per group
HSPA5 mRNA and protein response to PAH challenge
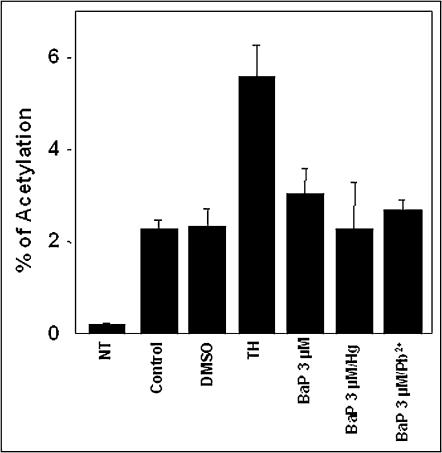
To determine whether positive regulation of HSPA5 was solely a response to heavy metals, rGMCs were challenged with BaP, a PAH that causes cellular stress in rGMCs via oxidative mechanisms (Bowes et al 1996). Treatment of rGMCs with 3 μM BaP increased HSPA5 mRNA, and this response was comparable to that elicited by TH (Fig 4). Like TH, the BaP response was AD sensitive, suggesting a role for transcriptional mechanisms in this response. Cotreatment of rGMCs with BaP and HgCl2 or Pb2+ acetate (1 μM) did not enhance induction response. Interestingly, BaP alone or in combination with heavy metals did not increase promoter activity (Fig 5).
Fig 4.
HSPA5 protein expression profiles in benzo(a)pyrene-stressed rat glomerular mesangial cells (rGMCs). Western blot analysis of HSPA5 protein in rGMCs exposed to 10 μM actinomycin (AD), 100 nM thapsigargin (TH), AD + TD, 3 μM BaP, 3 μM BaP + 10 μM AD, 3 μM BaP + 10 μM HgCl2, 3 μM BaP + 10 μM Pb2+ acetate
Fig 5.
Transcriptional activation profiles in metal-stressed rat glomerular mesangial cells (rGMCs). HSPA5–chloramphenicol acetyltransferase (CAT) constructs were transiently transfected into rGMCs and CAT activity was measured as described in Materials and Methods. CAT activities were measured in cells challenged with dimethyl sulfoxide, 100 nM TH, 3 μM BaP, BaP + 3 μM HgCl2, or 3 μM BaP + 3 μM Pb2+ acetate. Similar results were seen in 3 separate experiments with at least 3 replicate cultures per group
The HSPA5 induction response to both TH and BaP was conserved because it was seen in M15 and HEK293 cells, lines of embryonic murine and human origin (Fig 6A–C). In these experiments, exposure of M15 cells to 3 and 30 μM BaP induced HSPA5 in a dose-dependent manner similar to TH and consistent with the induction seen in HEK293 cells (Baqui et al 2000) and evidenced by significant increases in HSPA5 positive objects and area (Fig 6B,C). The use of M15 cells was advantageous because their morphology allowed for optimal localization of the HSPA5 signal.
Fig 6.
Immunocytochemical localization of HSPA5 in M15 and HEK293 cells. Images represent data taken from 3 different experiments in which 20 images (∼30 cells/image) per biological triplicate were analyzed for HSPA5 signal, corrected for a no–primary antibody background subtraction, and compared with vehicle control. (A) Representative images from HSPA5; (B, C) quantification
To identify evolutionarily conserved sequences within the HSPA5 promoter that function as putative transcription factor binding sites activated by cellular stress, DNA sequences consisting of 2.7 Kb upstream of the +1 position of the HSPA5 gene from mouse and rat were analyzed with DiAlign and MatInspector software. These sites are schematically illustrated in Figure 7. Of note is the presence of HEN1, NFY, and NFκB binding sites within 250 bp of the construct employed in reporter experiments.
Fig 7.
Schematic representation of the conserved stress-associated putative transcription factor binding sites in the mouse and rat HSPA5 gene promoters. Dark regions represent sequences with high homology between the 2 species. Clear areas represent regions in which no matching was found. Arrows indicate the direction in which the sequence is located. Transcription factor binding sites are depicted to an approximate scale
DISCUSSION
HSPA5 functions as an ER stress sensor triggering the UPR, a highly conserved adaptive response designed to act as a molecular chaperone and calcium buffering system, as well as a key member in PERK-eIF2α–mediated attenuation of protein synthesis (Pain 1996; Bertolotti et al 2000). Down-regulation of the UPR response leads to ER stress, as seen in neurodegenerative diseases such as Alzheimer's (Imaizumi et al 2001) and Parkinson's disease (Imai et al 2000). In previous studies we have shown that HgCl2 and Pb2+ acetate at the same concentrations examined in this study induce HSPA5 mRNA and protein and facilitate nonhomogeneous cellular distribution in rat C6 glioma cells (Qian et al 2005a, 2005b). In addition, dsRNAi oligo-mediated HSPA5 degradation–induced reactive oxygen species, resulting from Pb2+ exposure, suggests a mechanism for Pb2+-mediated neurotoxicity via direct interactions with HSPA5 (Qian et al 2005a). Evidence is presented here that rGMCs exhibit differential patterns of metal and hydrocarbon-specific regulation of HSPA5 and involves transcriptional as well as posttranscriptional mechanisms. These findings are consistent with previous work by others implicating ATF4, ATF1, and CREB1 in transcriptional (Li et al 1997) as well as posttranscriptional control of HSPA5 (Lam et al 1992; Ulatowski et al 1993).
In rGMCs, Pb2+ acetate increases HSPA5 mRNA and protein levels via transcriptional, AD-sensitive mechanisms that are distinct from those that mediate TH inducibility. This conclusion is based on the finding that both mRNA and protein accumulation were AD sensitive, but not mediated via the same regulatory elements that control TH inducibility. Thapsigargin is known to dysregulate intracellular Ca2+ storage, resulting in induction of the ER stress response characterized by increases in HSPA5 protein (Prostko et al 1992). Of interest was the finding that accumulation of HSPA5 mRNA in cells treated with HgCl2 was not AD-sensitive, whereas protein accumulation was both AD and Cyc sensitive. These results suggest that transcriptional regulators of HSPA5 can themselves be regulated at the transcriptional and posttranscriptional level. Tully et al (2000) have shown modest, dose-dependent inductions of HSPA5 promoter activity in HepG2 cells treated with lead nitrate at doses higher than 12.5 μM. This induction is consistent with previous reports by Shelton et al (1986) showing that lead glutamate induces HSPA5 in kidney epithelial cells. Although the inability of HgCl2 to induce HSPA5 mRNA in rGMCs via a transcriptional mechanism might reflect our use of low metal concentrations, higher concentrations of HgCl2 did not influence HSPA5 inducibility in transient reporter assays (not shown), suggesting that transcriptional activation is not involved in the regulation of HSPA5 mRNA levels by HgCl2. It is important to note that concentrations of HgCl2 and Pb2+ acetate higher than 10 μM are not representative of those accumulated by living organisms in the natural environment (Sharma and Agrawal 2005; Florea and Busselberg 2006). In fact, high concentrations of HgCl2 and Pb2+ acetate cause a significant decrease in cell viability that precludes interpretation of any gene regulation findings. The fact that AD did not inhibit the induction of HSPA5 by HgCl2 implicates posttranscriptional mechanisms in the regulation of HSPA5 by this metal.
In vitro concentrations of 1 μM HgCl2 and Pb2+ acetate are equivalent to levels of 275 μg/L and 40 μg/dL in blood, respectively. Both Hg2+ and Pb2+ are highly lipophilic metals that cross the blood-brain and placental barriers, readily distributing throughout the body and accumulating primarily in the kidneys. Organic mercury compounds distribute throughout the body and are most efficient with respect to kidney accumulation (ATSDR 1999). Mercury exposures of 50 μg/L in blood or 20 μg/L in urine must be disclosed in the occupational Heavy Metals Registry of various states. The usual toxic threshold for Hg in blood is 50 μg/L, with normal considered to be <10 μg/L (Blayney 2001). The World Health Organization has reported that populations with high fish consumption can have blood methylmercury levels of 200 μg/L, a concentration associated with a 5% risk of neurological damage to adults (WHO, 1990). In populations eating certain quantities of fish the Hg blood concentration ranged from 32.7 to 44.6 in mothers and 51.4 to 140 μg/L in children (Hansen et al 1991). Employees in the alkali and chlorine manufacturing industry have shown maximal blood Hg concentrations of 916 μg/L, whereas manufacturing of industrial instruments results in exposures averaging blood Hg concentrations of 145 μg/L, with maximal exposures of 889 μg/L (Baser and Marion 1990).
Lead nephrotoxicity involves proximal tubular nephropathy, glomerular sclerosis, and interstitial fibrosis (Goyer 1989; Loghman-Adham 1997; Diamond 2005). Morphological studies have shown that lead exposure results in the formation of intranuclear inclusion bodies, cellular necrosis in the proximal tubule, and interstitial fibrosis (Cramer et al 1974; Wedeen et al 1975; Biagini et al 1977). Enzymuria and proteinuria were detected in humans with 20–50 μg/dL exposure (Wedeen et al 1975, 1979; dos Santos et al 1994; Verberk et al 1996). Severe functional deficits, including enzymuria, proteinuria, impaired transport, and depressed glomerular filtration rate, occur at exposures >50 μg/dL (Lilis et al 1968; Baker et al 1980; Hong et al 1980; ATSDR, 2005). As described for the heavy metals, BaP exposures at 3 μM span the range of estimated human exposures 0.171–1.64 μg/d (Busbee et al 1990; USEPA 1990; Bral and Ramos 1997).
Previous studies in this laboratory have shown that BaP induces DNA adducts in glomerular cells in vitro (Bowes et al 1996) and inhibits c-fos, c-jun, and c-Ha-ras expression (Parrish et al 1998). BaP significantly induces HSPA5 mRNA in rGMCs, and this response is inhibited by AD (Fig 4). PAHs have been shown to have a TH-like effect on ER calcium ATPase (Krieger et al 1995), but the HSPA5 inducibility profiles elicited by BaP differed from those by TH, suggesting that deficits in calcium homeostasis are not involved in the HSPA5 response to BaP.
The finding that binary mixtures of mercuric chloride or lead acetate with BaP did not influence HSPA5 expression suggests that rGMCs exhibit differential patterns of metal-specific gene regulation compared with BaP. Therefore, distinct mechanisms of cellular adaptation can govern the response to different forms of chemical stress. In this scenario, the transcriptional regulatory response triggered by metals could involve recruitment and assembly of macromolecular complexes distinct from those activated by BaP. This suggestion is consistent with previous reports showing that BaP activates DNA binding by the aryl hydrocarbon receptor (AhR) transcription factor, whereas heavy metals inhibit this response (Wei et al 2004). Likewise, metals can activate stress response elements not affected by BaP, as shown in the regulation of heme oxigenase-1 (HO-1, encoded by Hmox1) gene expression in murine hepatoma Hepa 1c1c7 cells (Korashy and El-Kadi 2004).
It should be noted, however, that Hg and Pb can induce mRNA expression of AhR-regulated genes via AhR–xenobiotic response element (XRE) interactions, Nrf2-XRE interactions, or both (Degawa et al 1994; Korashy and El-Kadi 2004, 2006a, 2006b). In hepatic cell lines, BaP and heavy metals induce HSPA5 mRNA expression via common response elements that saturate on cotreatment, resulting in lack of a cumulative HSPA5 response (Korashy and El-Kadi 2004, 2006a). If this was the case in rGMCs, the inducible range for HSPA5 would be narrow and saturable at reasonably low doses of heavy metals in combination with BaP.
To determine whether this response is conserved in renal cells, we monitored HSPA5 expression profiles in M15 and HEK293 cells, murine and human lines of embryonic origin, respectively (Graham et al 1977; Larsson et al 1995). The data indicate that 3 μM BaP elicited a heterogeneous pattern of HSPA5 protein induction and mobilization in M15 cells that is different from TH, supporting the conclusion that BaP-induced regulation of HSPA5 differs from classical ER stressors, such as TH, or from heavy metals.
Perturbations of Ca2+ homeostasis, redox status, protein phosphorylation, or a combination of factors could be responsible for the modulation of HSPA5 expression in rGMCs by chemical injury. Critical regulatory sequences have been identified in the region spanning nucleotides from −378 to −1 of the rat promoter (Chang et al 1987). Sequence analysis identified several putativexenobiotic-regulatory sequences—namely, ARE/EpRE-, CCAAT-, CRE-, and TRE-like—within this region. Measurements of transactivation potential with the use of CAT-reporter constructs with these sequences and comparison to a series of promoter deletion mutants was employed to evaluate molecular mechanisms of HSPA5 regulation. Transient transfection experiments showed that BaP, HgCl2, and Pb2+ acetate do not induce HSPA5 reporter activity in rGMCs. However, RT-PCR measurements revealed that BaP induced HSPA5 transcription and implicated a transcriptional mechanism in this response. Thus, the HSPA5 promoter construct employed in our studies is likely missing critical regulatory elements required for transcriptional regulation by heavy metals or BaP.
Analysis of the complete HSPA5, 5′-UTR identified several putative binding sites for transcriptional regulators activated by cellular stress. Of note is the presence of HEN1, NFY, and NFκB binding sites within 250 base pairs of the construct employed in reporter experiments. Hu et al (2006) have shown that ER stress by TH and tunicamycin activates NFκB via an IRE1α-dependent mechanism. NFκB might in fact play a dual role in the regulation of TNFα-mediated apoptosis and the up-regulation of ER stress genes depending on the level of stress (Hung et al 2004; Hu et al 2006). Ubeda and Habener (2000) identified a short region in the CHOP reporter conserved in GRP78, GRP94, PDI, and calreticulin that is constitutively regulated by NFY and necessary for ER stress-mediated assembly of the CHOP transcriptional complex.
Gulow et al (2002) have shown that in stably transfected HeLa Bi11 cells, HSPA5 is controlled at the posttranscriptional level and feedback regulated by protein translation efficiency. Although artificial increases in mRNA do not increase protein levels in unstressed Bi11 cells, under ER stress conditions, and independent of transcript levels, the translational efficiency of HSPA5 increases. Attenuation of protein translation is mediated through the PERK-eIF2α. ATF4, a transcription factor in which translation is up-regulated by the PERK-eIF2α pathway, can activate the HSPA5 promoter via an ATF/CRE sequence located between −190 and −183 of the promoter. When ATF4 function is suppressed genetically, HSPA5 induction by TH or tunicamycin is partially inhibited (Luo et al 2003). Additional studies to evaluate DNA/protein interactions in the regulation of HSPA5 gene warrant further investigation. Recent work has suggested that HSPA5 regulation can occur at the translational level (Gulow et al 2002) and involve modulation of internal ribosomal entry sites (Li et al 1997; Kimata et al 2003). Although this is the first report of HSPA5 promoter activity in any renal cell line, the results suggest that transcriptional and posttranscriptional regulation of this gene varies according to cell type and form of stress.
REFERENCES
- [ATSDR] Agency for Toxic Substances and Disease Registry. 1999 Toxicological Profile for Mercury. Department of Human Health Services, Washington, DC. [PubMed] [Google Scholar]
- ATSDR. 2005 Toxicological Profile for Lead. Department of Human Health Services. Washington, DC. [Google Scholar]
- Baker EL Jr, Goyer RA, and Fowler BA. et al. 1980 Occupational lead exposure, nephropathy, and renal cancer. Am J Ind Med. 1:139–148. [DOI] [PubMed] [Google Scholar]
- Baqui MMA, Gereben B, Harney JW, Larsen PR, Bianco AC. Distinct subcellular localization of transiently expressed types 1 and 2 Iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology. 2000;141:4309–4312. doi: 10.1210/endo.141.11.7872.0013-7227(2000)141[4309:DSLOTE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Baser ME, Marion D. A statewide case registry for surveillance of occupational heavy metals absorption. Am J Public Health. 1990;80:162–164. doi: 10.2105/ajph.80.2.162.1541-0048(1990)080[0162:ASCRFS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister P, Luo S, Skarnes WC, Sui G, Seto E, Shi Y, Lee AS. Endoplasmic reticulum stress induction of the Grp78/BiP promoter: activating mechanisms mediated by YY1 and its interactive chromatin modifiers. Mol Cell Biol. 2005;25:4529–4540. doi: 10.1128/MCB.25.11.4529-4540.2005.1098-5549(2005)025[4529:ERSIOT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014.1465-7392(2000)002[0326:DIOBAE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Biagini G, Misciattelli ME, Contri Baccarani M, Vangelista A, Raffi GB, and Caudarella R 1977 [Electron microscopy features of renal changes in chronic lead poisoning]. Lav Um. 29:179–187.[in Italian]. [PubMed] [Google Scholar]
- Blayney MB. The need for empirically derived permeation data for personal protective equipment: the death of Dr. Karen E. Wetterhahn. Appl Occup Environ Hyg. 2001;16:233–236. doi: 10.1080/104732201460389.1047-322X(2001)016[0233:TNFEDP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bowes I, Russell C, and Parrish AR. et al. 1996 Atypical cytochrome P450 induction profiles in glomerular mesangial cells at the mRNA and enzyme level : evidence for CYP1A1 and CYP1B1 expression and their involvement in benzo[a]pyrene metabolism. Biochem Pharmacol. 52:587–595. [DOI] [PubMed] [Google Scholar]
- Bral CM, Ramos KS. Identification of benzo[a]pyrene-inducible cis-acting elements within c-Ha-ras transcriptional regulatory sequences. Mol Pharmacol. 1997;52:974–982. doi: 10.1124/mol.52.6.974.0026-895X(1997)052[0974:IOBCEW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brooks DA. Protein processing: a role in the pathophysiology of genetic disease. FEBS Lett. 1997;409:115–120. doi: 10.1016/s0014-5793(97)00423-7.0014-5793(1997)409[0115:PPARIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Busbee DL, Norman JO, Ziprin RL. Comparative uptake, vascular transport, and cellular internalization of aflatoxin-B1 and benzo(a)pyrene. Arch Toxicol. 1990;64:285–290. doi: 10.1007/BF01972988.0340-5761(1990)064[0285:CUVTAC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chang SC, Wooden SK, and Nakaki T. et al. 1987 Rat gene encoding the 78-kDa glucose-regulated protein GRP78: its regulatory sequences and the effect of protein glycosylation on its expression. PNAS. 84:680–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer K, Goyer RA, Jagenburg R, Wilson MH. Renal ultrastructure, renal function, and parameters of lead toxicity in workers with different periods of lead exposure. Br J Ind Med. 1974;31:113–127. doi: 10.1136/oem.31.2.113.0007-1072(1974)031[0113:RURFAP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degawa M, Arai H, Kubota M, Hashimoto Y. Ionic lead, a unique metal ion as an inhibitor for cytochrome P450IA2 (CYP1A2) expression in the rat liver. Biochem Biophys Res Commun. 1994;200:1086–1092. doi: 10.1006/bbrc.1994.1561.0006-291X(1994)200[1086:ILAUMI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dhakshinamoorthy S, Long DJ 2nd, Jaiswal AK. Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Curr Top Cell Regul. 2000;36:201–216. doi: 10.1016/s0070-2137(01)80009-1.0070-2137(2000)036[0201:AROGEE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Diamond GL 2005 Risk assessment of nephrotoxic metals. In: The Toxicology of the Kidney, ed Tarloff J, Lash L. CRC Press, London, 1099–1132. [Google Scholar]
- dos Santos AC, Colacciopo S, Dal Bo CM, dos Santos NA. Occupational exposure to lead, kidney function tests, and blood pressure. Am J Ind Med. 1994;26:635–643. doi: 10.1002/ajim.4700260506.0271-3586(1994)026[0635:OETLKF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Florea AM, Busselberg D. Occurrence, use and potential toxic effects of metals and metal compounds. Biometals. 2006;19:419–427. doi: 10.1007/s10534-005-4451-x.0966-0844(2006)019[0419:OUAPTE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gething M-J. Role and regulation of the ER chaperone BiP*1. Sem Cell Devel Biol. 1999;10:465–472. doi: 10.1006/scdb.1999.0318.1084-9521(1999)010[0465:RAROTE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Goyer RA. Mechanisms of lead and cadmium nephrotoxicity. Toxicol Lett. 1989;46:153–162. doi: 10.1016/0378-4274(89)90124-0.0378-4274(1989)046[0153:MOLACN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Goyer RA, Rhyne BC. Pathological effects of lead. Int Rev Exp Pathol. 1973;12:1–77.0074-7718(1973)012[0001:PEOL]2.0.CO;2 [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59.0022-1317(1977)036[0059:COAHCL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gulow K, Bienert D, Haas IG. BiP is feed-back regulated by control of protein translation efficiency. J Cell Sci. 2002;115:2443–2452. doi: 10.1242/jcs.115.11.2443.0021-9533(2002)115[2443:BIFRBC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hansen JC, Jensen TG, and Tarp U 1991 Changes in blood mercury and lead levels in pregnant women in Greenland 1983–1988. Arctic Med Res. x(Suppl). 605–607. [PubMed] [Google Scholar]
- Henry GA, Jarnot BM, Steinhoff MM, Bigazzi PE. Mercury-induced renal autoimmunity in the MAXX rat. Clin Immunol Immunopathol. 1988;49:187–203. doi: 10.1016/0090-1229(88)90109-2.0090-1229(1988)049[0187:MRAITM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hirszel P, Michaelson JH, Dodge K, Yamase H, Bigazzi PE. Mercury-induced autoimmune glomerulonephritis in inbred rats. II. Immunohistopathology, histopathology and effects of prostaglandin administration. Surv Synth Pathol Res. 1985;4:412–422.0253-438X(1985)004[0412:MAGIIR]2.0.CO;2 [PubMed] [Google Scholar]
- Hong CD, Hanenson IB, Lerner S, Hammond PB, Pesce AJ, Pollak VE. Occupational exposure to lead: effects on renal function. Kidney Int. 1980;18:489–494. doi: 10.1038/ki.1980.162.0085-2538(1980)018[0489:OETLEO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1{alpha}-mediated NF-{kappa}B activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006.1098-5549(2006)026[3071:ATNFAL]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung J-H, Su I-J, and Lei H-Y. et al. 2004 Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-{kappa}B and pp38 mitogen-activated protein kinase. J Biol Chem. 279:46384–46392. [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275:35661–35664. doi: 10.1074/jbc.C000447200.0021-9258(2000)275[35661:PSUPSC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Imaizumi K, Miyoshi K, Katayama T, Yoneda T, Taniguchi M, Kudo T, Tohyama M. The unfolded protein response and Alzheimer's disease. Biochim Biophys Acta Mol Basis Dis. 2001;1536:85–96. doi: 10.1016/s0925-4439(01)00049-7.0925-4439(2001)1536[0085:TUPRAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kimata Y, Kimata YI, and Shimizu Y. et al. 2003 Genetic evidence for a role of BiP/Kar2 that regulates Ire1 in response to accumulation of unfolded proteins. Mol Biol Cell. 14:2559–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korashy HM, El-Kadi AO. Differential effects of mercury, lead and copper on the constitutive and inducible expression of aryl hydrocarbon receptor (AHR)–regulated genes in cultured hepatoma Hepa 1c1c7 cells. Toxicology. 2004;201:153–172. doi: 10.1016/j.tox.2004.04.011.0300-483X(2004)201[0153:DEOMLA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Korashy HM, El-Kadi AO. The role of aryl hydrocarbon receptor and the reactive oxygen species in the modulation of glutathione transferase by heavy metals in murine hepatoma cell lines. Chem Biol Interact. 2006a;162:237–248. doi: 10.1016/j.cbi.2006.07.002.0009-2797(2006)162[0237:TROAHR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Korashy HM, El-Kadi AO. Transcriptional regulation of the NAD(P)H:quinone oxidoreductase 1 and glutathione S-transferase ya genes by mercury, lead, and copper. Drug Metab Dispos. 2006b;34:152–165. doi: 10.1124/dmd.105.005397.0090-9556(2006)034[0152:TROTNO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Krieger JA, Davila DR, Lytton J, Born JL, Burchiel SW. Inhibition of sarcoplasmic/endoplasmic reticulum calcium ATPases (SERCA) by polycyclic aromatic hydrocarbons in HPB-All human T cells and other tissues. Toxicol Appl Pharmacol. 1995;133:102–108. doi: 10.1006/taap.1995.1131.0041-008X(1995)133[0102:IOERCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lam M, Vimmerstedt LJ, Schlatter LK, Hensold JO, Distelhorst CW. Preferential synthesis of the 78-Kd glucose-regulated protein in glucocorticoid-treated S49 mouse lymphoma cells. Blood. 1992;79:3285–3292.0006-4971(1992)079[3285:PSOTKG]2.0.CO;2 [PubMed] [Google Scholar]
- Larsson SH, Charlieu JP, and Miyagawa K. et al. 1995 Subnuclear localization of WT1 in splicing or transcription factor domains is regulated by alternative splicing. Cell. 81:391–401. [DOI] [PubMed] [Google Scholar]
- Li W, Hsiung Y, Zhou Y, Roy B, Lee A. Induction of the mammalian GRP78/BiP gene by Ca2+ depletion and formation of aberrant proteins: activation of the conserved stress-inducible grp core promoter element by the human nuclear factor YY1. Mol Cell Biol. 1997;17:54–60. doi: 10.1128/mcb.17.1.54.1098-5549(1997)017[0054:IOTMBG]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilis R, Gavrilescu N, Nestorescu B, Dumitriu C, Roventa A. Nephropathy in chronic lead poisoning. Br J Ind Med. 1968;25:196–202. doi: 10.1136/oem.25.3.196.0007-1072(1968)025[0196:NICLP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr. 1994;4:1–18. doi: 10.1615/critreveukargeneexpr.v4.i1.10.1045-4403(1994)004[0001:TGPGAG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liu J-S, Kuo S-R, Makhov AM, Cyr DM, Griffith JD, Broker TR, Chow LT. Human Hsp70 and Hsp40 chaperone proteins facilitate human papillomavirus-11 E1 protein binding to the origin and stimulate cell-free DNA replication. J Biol Chem. 1998;273:30704–30712. doi: 10.1074/jbc.273.46.30704.0021-9258(1998)273[30704:HHAHCP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Loghman-Adham M. Renal effects of environmental and occupational lead exposure. Environ Health Perspect. 1997;105:928–939. doi: 10.1289/ehp.97105928.0091-6765(1997)105[0928:REOEAO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Baumeister P, Yang S, Abcouwer SF, Lee AS. Induction of Grp78/BiP by translational block: activation of the Grp78 promoter by ATF4 through and upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J Biol Chem. 2003;278:37375–37385. doi: 10.1074/jbc.M303619200.0021-9258(2003)278[37375:IOBBTB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Magos L, Sparrow S, Snowden RT. Effect of prolonged saline loading on HgCl2-induced renal tubular damage. Br J Exp Pathol. 1984;65:567–575.0959-9673(1984)065[0567:EOPSLO]2.0.CO;2 [PMC free article] [PubMed] [Google Scholar]
- Mao C, Tai W-C, Bai Y, Poizat C, Lee AS. In vivo regulation of Grp78/BiP transcription in the embryonic heart: role of the endoplasmic reticulum stress response element and GATA-4. J Biol Chem. 2006;281:8877–8887. doi: 10.1074/jbc.M505784200.0021-9258(2006)281[8877:IVROBT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/s0143416002001884.0143-4160(2002)032[0269:CSACBC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pain VM. Initiation of protein synthesis in eukaryotic cells. Eur J Biochem. 1996;236:747–771. doi: 10.1111/j.1432-1033.1996.00747.x.0014-2956(1996)236[0747:IOPSIE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Parrish A, Alejandro N, Bowes R III, Ramos K. Cytotoxic response profiles of cultured renal epithelial and mesenchymal cells to selected aromatic hydrocarbons. Toxicol In Vitro. 1998;12:219–232. doi: 10.1016/s0887-2333(97)00118-5.0887-2333(1998)012[0219:CRPOCR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Prostko CR, Brostrom MA, Malara EM, Brostrom CO. Phosphorylation of eukaryotic initiation factor (eIF) 2 alpha and inhibition of eIF-2B in GH3 pituitary cells by perturbants of early protein processing that induce GRP78. J Biol Chem. 1992;267:16751–16754.0021-9258(1992)267[16751:POEIFE]2.0.CO;2 [PubMed] [Google Scholar]
- Qian Y, Falahatpisheh MH, Zheng Y, Ramos KS, Tiffany-Castiglioni E. Induction of 78 kD glucose-regulated protein (GRP78) expression and redox-regulated transcription factor activity by lead and mercury in C6 rat glioma cells. Neurotox Res. 2001;3:581–589. doi: 10.1007/BF03033212.1029-8428(2001)003[0581:IOKGPG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Qian Y, Mikeska G, Harris ED, Bratton GR, Tiffany-Castiglioni E. Effect of lead exposure and accumulation on copper homeostasis in cultured C6 rat glioma cells. Toxicol Appl Pharmacol. 1999;158:41–49. doi: 10.1006/taap.1999.8657.0041-008X(1999)158[0041:EOLEAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Qian Y, Tiffany-Castiglioni E, Harris ED. Copper transport and kinetics in cultured C6 rat glioma cells. Am J Physiol. 1995;269:C892–C898. doi: 10.1152/ajpcell.1995.269.4.C892.0002-9513(1995)269[C892:CTAKIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Qian Y, Zheng Y, Ramos KS, Tiffany-Castiglioni E. GRP78 compartmentalized redistribution in Pb-treated glia: role of GRP78 in lead-induced oxidative stress. Neurotoxicology. 2005a;26:267–275. doi: 10.1016/j.neuro.2004.09.002.0161-813X(2005)026[0267:GCRIPG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Qian Y, Zheng Y, Ramos KS, Tiffany-Castiglioni E. The involvement of copper transporter in lead-induced oxidative stress in astroglia. Neurochem Res. 2005b;30:429–438. doi: 10.1007/s11064-005-2677-1.0364-3190(2005)030[0429:TIOCTI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rao RV, Peel A, and Logvinova A. et al. 2002 Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 514:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez EJ, Wooden SK, Lee AS. Identification of highly conserved regulatory domains and protein-binding sites in the promoters of the rat and human genes encoding the stress-inducible 78-kilodalton glucose-regulated protein. Mol Cell Biol. 1988;8:4579–4580. doi: 10.1128/mcb.8.10.4579.1098-5549(1988)008[4579:IOHCRD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Li WW, Lee AS. Calcium-sensitive transcriptional activation of the proximal CCAAT regulatory element of the grp78/BiP promoter by the human nuclear factor CBF/NF-Y. J Biol Chem. 1996;271:28995–29002. doi: 10.1074/jbc.271.46.28995.0021-9258(1996)271[28995:CTAOTP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sharma RK, Agrawal M. Biological effects of heavy metals: an overview. J Environ Biol. 2005;26:301–313.0254-8704(2005)026[0301:BEOHMA]2.0.CO;2 [PubMed] [Google Scholar]
- Shelton KR, Todd JM, Egle PM. The induction of stress-related proteins by lead. J Biol Chem. 1986;261:1935–1940.0021-9258(1986)261[1935:TIOSPB]2.0.CO;2 [PubMed] [Google Scholar]
- Tully DB, Collins BJ, Overstreet JD, Smith CS, Dinse GE, Mumtaz MM, Chapin RE. Effects of arsenic, cadmium, chromium, and lead on gene expression regulated by a battery of 13 different promoters in recombinant HepG2 cells. Toxicol Appl Pharmacol. 2000;168:79–90. doi: 10.1006/taap.2000.9014.0041-008X(2000)168[0079:EOACCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ubeda M, Habener JF. CHOP gene expression in response to endoplasmic-reticular stress requires NFY interaction with different domains of a conserved DNA-binding element. Nucleic Acids Res. 2000;28:4987–4997. doi: 10.1093/nar/28.24.4987.0305-1048(2000)028[4987:CGEIRT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulatowski LM, Lam M, Vanderburg G, Stallcup MR, Distelhorst CW. Relationship between defective mouse mammary tumor virus envelope glycoprotein synthesis and GRP78 synthesis in glucocorticoid-treated mouse lymphoma cells. Evidence for translational control of GRP78 synthesis. J Biol Chem. 1993;268:7482–7488.0021-9258(1993)268[7482:RBDMMT]2.0.CO;2 [PubMed] [Google Scholar]
- [USEPA] US Environmental Protection Agency. 1990 Toxicological Profile for Polycyclic Aromatic Hydrocarbons. USEPA PB 91-181537, Washington, DC. [Google Scholar]
- Verberk MM, Willems TE, Verplanke AJ, De Wolff FA. Environmental lead and renal effects in children. Arch Environ Health. 1996;51:83–87. doi: 10.1080/00039896.1996.9935998.0003-9896(1996)051[0083:ELAREI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wedeen RP, Maesaka JK, Weiner B, Lipat GA, Lyons MM, Vitale LF, Joselow MM. Occupational lead nephropathy. Am J Med. 1975;59:630–641. doi: 10.1016/0002-9343(75)90224-7.0002-9343(1975)059[0630:OLN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wedeen RP, Malik DK, Batuman V. Detection and treatment of occupational lead nephropathy. Arch Intern Med. 1979;139:53–57.0003-9926(1979)139[0053:DATOOL]2.0.CO;2 [PubMed] [Google Scholar]
- Wei YD, Tepperman K, Huang MY, Sartor MA, Puga A. Chromium inhibits transcription from polycyclic aromatic hydrocarbon-inducible promoters by blocking the release of histone deacetylase and preventing the binding of p300 to chromatin. J Biol Chem. 2004;279:4110–4119. doi: 10.1074/jbc.M310800200.0021-9258(2004)279[4110:CITFPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- [WHO] World Health Organization. 1990 Biennial Report of the Director-General to the World Health Assembly and to the United Nations. WHO, Geneva, Switzerland. [Google Scholar]
- Wooden SK, Li LJ, Navarro D, Qadri I, Pereira L, Lee AS. Transactivation of the grp78 promoter by malfolded proteins, glycosylation block, and calcium ionophore is mediated through a proximal region containing a CCAAT motif which interacts with CTF/NF-I. Mol Cell Biol. 1991;11:5612–5623. doi: 10.1128/mcb.11.11.5612.1098-5549(1991)011[5612:TOTGPB]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]