Abstract
Studies suggest that heat shock proteins (Hsps), Hsp70 in particular, may play a role in embryogenesis and reproduction. As the first trimester is the critical period of human fetal development, we tested whether there is an association between Hsp70 expression in lymphocytes and adverse pregnancy outcomes (APOs) during that period. We measured lymphocyte Hsp70 levels by immunoblot in 55 pregnant women with APOs and 110 well-matched controls selected from 778 pregnant women in a nested case-control study. Multivariate logistic regression models were used to investigate the association between lymphocyte Hsp70 levels and risk of developing APOs. Our data showed that Hsp70 levels in women with APOs, especially those younger than 29 years old, were significantly higher than controls (193 vs 135 units, P < 0.001) and that the elevated Hsp70 levels were associated with a significantly increased risk of APOs (adjusted OR = 1.014; 95% CI = 1.008–1.020, P < 0.001). Our results also showed that the sensitivity, specificity, and positive and negative predictive values were 78%, 60%, 50%, and 85%, respectively, among these pregnant women. Adjusted ORs and 95% CI for the association between a Hsp70 value > 153 IOD and APOs were statistically significant (OR = 8.78, 95% CI = 2.79–27.64, P < 0.001). These results suggest that Hsp70 may play a role in the etiology of APOs. However, the underlying mechanisms for the elevation of Hsp70 in women with APOs and whether Hsp70 can be applied as a clinical indicator of APOs warrant further investigations.
INTRODUCTION
Adverse pregnancy outcomes (APOs) are a group of common obstetric diseases, including abortions, dead fetus, dead delivery, preterm delivery, abnormalities, and intrauterine growth restriction, all of which are far more frequent in the developing world (Kramer 2003). Although APOs are important for both mother and infant, their etiology has long remained obscure. Many studies have recently been conducted in an effort to clarify risk factors for APOs. Thus, APOs have been reported to be associated with maternal psychological, social, physiological, pathological, and environmental stresses, such as smoking, alcohol use, and infection (Gibbs 2001; Wadhwa et al 2001; Cogswell et al 2003; Silbergeld and Patrick 2005). It is well known that these risk factors can induce the synthesis of a group of highly conserved proteins, called heat shock proteins (Hsps). Hsps function as intracellular molecular chaperones by participating in folding and by facilitating synthesis, assembly, and intracellular trafficking of proteins (Hightower 1991; Bukau and Horwich 1998). Hsps, particularly Hsp70, are rapidly and abundantly up-regulated to protect cells, organs, and living organisms from damage in response to an array of stresses, including hyperthermia, inflammation, infection, chemicals such as ethanol, and exposure to numerous xenobiotics (Welch 1992; Currie et al 1993; Wu et al 1996; Plumier et al 1997; Benjamin and McMillan 1998; Beck et al 2000; Xiao et al 2002, 2003; Mehta et al 2005). Hsp70 has also been found as an extracellular protein either expressed at the cell surface or free in plasma, where it can influence the immune system as suggested by numerous reports on the presence of autoantibodies to these proteins (reviewed in Tanguay and Wu 2006; Wu and Tanguay 2006).
Interestingly, Hsp70 has been shown to play an important role in cellular differentiation and embryonic development in mammals (Luft and Dix 1999; Christians et al 2003), and recent studies suggested an association between a previous infection and immunity to Hsps and reproductive failure or birth defects (Neuer et al 2000; Child et al 2006). Hsp70 is a potentially quantitative indicator of environmental stress and toxicity in human cells (Delmas et al 1995) and in humans (Xiao et al 2002, 2003); therefore, Hsp70 has served as biomarkers for evaluating disease states (Wright et al 2000; Jin et al 2004b) and damage resulting from exposure to environmental stresses (Xiao et al 2002, 2003). It is thought that human development undergoes a defined but adjustable program, depending on the plasticity of embryonic cell response to physiological and environmental changes, and the first trimester is the critical period of fetal development. Although changes of microenvironment within the mother are believed to influence fetal development and growth through exchanges in placenta and blood flow, few studies have investigated the role of maternal microenvironment changes in fetal development in humans, and no study has investigated the role of Hsps in APOs.
Because human lymphocytes are frequently used as the surrogate tissue to investigate association between protein expression in studies of diseases and cellular response to environmental stresses (Bonassi and Au 2002), we therefore hypothesized that the lymphocyte Hsp70 levels in early pregnancy women might be associated with APOs, and this was tested in a prospective nested case-control study.
MATERIALS AND METHODS
Subjects
The study population comprised 778 pregnant women in Wuhan between 1 May and 31 December 2003 who went to Hubei Hospital of Maternal Health Care for routine checkup in the first trimester and consented to participate in this prospective study. These women had been pregnant for a period of 4 to16 weeks. The routine checkup included questionnaire, full physical examination (eg, weight, height, signs, blood pressure, heart rate, oral temperature, electrocardiogram, and B echogram), and laboratory tests (ie, blood and urine tests, infection test including toxoplasma, rubellavirus, cytomegalovirus, herpes simplex virus, hepatitis B antigen, and their antibodies). The detailed questionnaire consisted of 50 items including age of both pregnant woman and her husband, ethnicity, educational and marital status, active or/and passive smoking (prenatal active and/or passive tobacco smoke exposure), alcohol consumption, living and working environments, and medical history (ie, previous infection and diseases and maternal psychological status). All participants were followed up for pregnancy outcomes that were diagnosed by Hubei Hospital of Maternal Health Care. There were 74 women with APOs by the end of October 2004. After exclusion of pregnant women without blood samples, there were 55 women with APOs, including 19 natural abortions, 14 induced abortions, 11 dead fetuses, 1 dead delivery, 4 preterm deliveries, 5 abnormalities (1 fetal defect and 4 malformations), and 1 intrauterine growth restriction, who are referred to as the case group. All induced abortions were for medical reason because of infections (5), fever of unknown origins (4), injury (1), radiation exposure (1), and contraindication for pregnant women (1). Each case was matched to 2 control subjects who were normal pregnant women without APOs according to age (least 3 years) and gestation period (1 week). The Ethics Committees of Tongji Medical College approved the study and all subjects provided their written, informed consent.
Blood sampling
To isolate lymphocytes, venous blood (∼5 ml) was drawn from each subject after overnight fasting. The collected blood was placed into a heparinized tube for isolating the lymphocytes using Ficoll-Hypaque (Biochemical Reagent Co, Shanghai, China) as previously described (Xiao et al 2002). The collected lymphocytes were washed twice with phosphate-buffered saline (PBS) and counted. The number of lymphocytes was adjusted to 5000/μL with PBS. Two hundred microliters of lymphocytes in PBS was centrifuged at room temperature and the buffer solution removed as soon as possible. Lymphocytes were stored at–80°C for further analysis.
Detection of Hsp70
Hsp70 levels in lymphocytes were determined as previously described (Xiao et al 2002) with minor modifications. Briefly, the lymphocytes were mixed with 1× sodium dodecyl sulfate (SDS) sample buffer and boiled at 100°C for 10 minutes, and equal numbers of cells from each subject were loaded onto each lane of SDS-polyacrylamide gel. After electrophoresis, proteins were transferred electrophoretically to nitrocellulose membranes, and the membranes were then saturated with blocking buffer (PBS containing 5% skim milk powder) for 1 hour at 37°C. The rabbit anti-human Hsp70 antibody specific for the inducible Hsp71 (#799 in Tanguay et al 1993) at a dilution 1:1000 in PBS containing 5% skim milk powder was incubated with the nitrocellulose membranes at 37°C for 1 hour with gentle agitation. After washing the membranes 6 times (10 minutes each time) with 200 μL PBS-0.05% Tween 80, horseradish peroxidase labeled goat anti-rabbit IgG in blocking buffer (1:1000) was incubated with nitrocellulose membranes at 37°C for another 1 hour. Membranes were washed 4 times with 200 μL PBS-0.05% Tween 80. The presence of Hsp70 on nitrocellulose membranes was revealed using DAB (3,3-diaminobenzidine tetrahydrochloride) buffer for 3–5 minutes. The relative signal intensity was quantified by densitometry with Gel pro3.0 image software (Media Cybernetics, Silver Spring, MD, USA) on an IBM-compatible personal computer, and the integrated optical density (IOD) was used to present the relative levels of Hsp70. The status of pregnancy outcome was blind to the experimenters.
Statistical analyses
All data were entered into a computerized database. Further analysis was carried out by using the statistical analysis software SPSS 12.0 package (SPSS Inc, Chicago, IL, USA). Measurements of continuous data were analyzed by univariate analysis of variance and independent sample Student t-tests. Qualitative data were computed by the Pearson χ2 contingency tables. Crude and adjusted odds ratios (ORs) with 95% confidence intervals (CI) were computed by multivariate logistic regression analysis to estimate the magnitude of associations between APOs risk and the Hsp70 levels. Statistical inference was based on the significance of P < 0.05.
RESULTS
Baseline characteristics of cases and controls
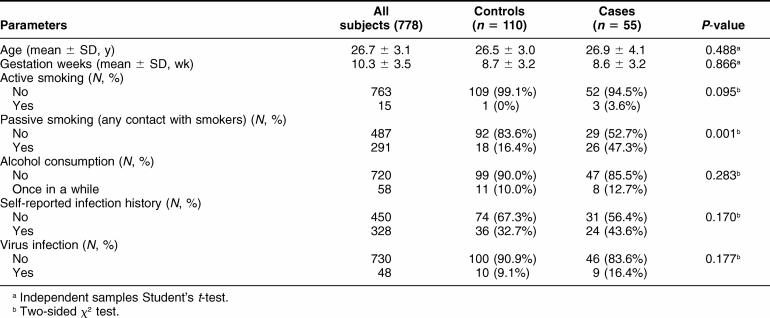
The characteristics of cases and controls are summarized in Table 1. Cases and controls were well matched for age and pregnancy weeks. There were no significant differences in the frequencies of active smoking, drinking, self-reported infection history, and virus infection among cases and controls, although the rates of self-reported infection history or virus infection were slightly higher in the case than in the control group (Table 1). The passive smoking was also significantly more common in cases than in controls (P = 0.001).
Table 1.
Basic characteristics of patients (cases) and controls
Lymphocyte Hsp70 levels in cases and controls
Figure 1 shows a graphic presentation of lymphocyte Hsp70 values for all individuals of both cases and controls. There was a large variation among Hsp70 levels in women of both groups, and the lymphocyte Hsp70 levels were decreased as age increased, especially in the cases group (Table 2). Table 2 lists the mean IOD of the lymphocyte Hsp70 levels of both cases and controls. The Hsp70 levels for all the cases were significantly higher than in the controls (193 vs 135 IOD, P < 0.001) (Table 2). Further analyses by stratification of age showed that there was a significantly higher level of Hsp70 in the age <25 (216 vs 147 IOD) and the 25–29 case group (198 vs 131 IOD) (P = 0.001 and P < 0.001) but not in the >29 case group (139 vs 130, P = 0.902) compared with the corresponding control group.
Fig 1.
Lymphocyte Hsp70 levels in cases and controls of different age-groups of pregnant women. Lymphocytes were isolated from 165 subjects aged between 19 and 39 years, and Hsp70 levels were determined by Western blot. The levels of Hsp70 are expressed in integrated optical density (IOD)
Table 2.
Lymphocyte Hsp70 levels and their association with adverse pregnancy in women of different age-groups
Association of Hsp70 levels with adverse pregnancy outcomes
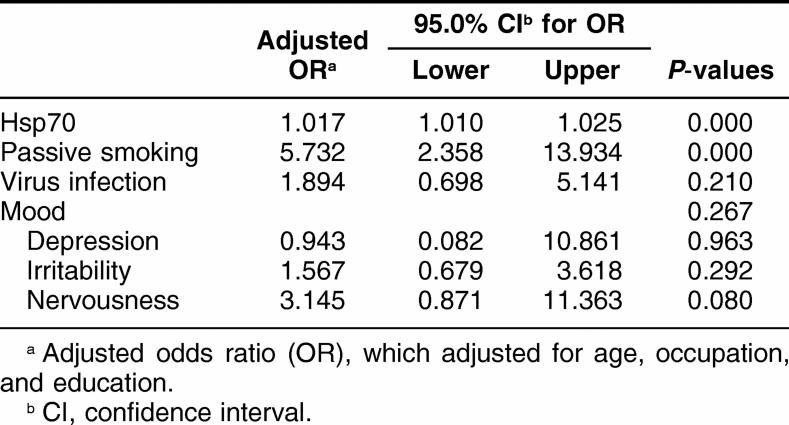
Because there were many risk factors that may influence pregnant outcomes, multiple logistic regression analysis was performed. As shown in Table 3, there was a significantly positive association of APOs with passive smoking and Hsp70 after adjustment for age, occupation, and educational status (P < 0.001). Further analysis showed that there was a significant positive correlation of lymphocyte Hsp70 levels in all early pregnant women with APOs (OR: 1.0014, 95% CI: 1.008–10020, P < 0.001) (Table 2). In addition, there was a significant correlations of lymphocyte Hsp70 levels in early pregnant women with APOs in the age <25 and 25–29 case groups (P = 0.05 and P < 0.001) but not in the >29 case group (P > 0.05) compared with the corresponding controls (Table 2).
Table 3.
Association of adverse pregnancy outcomes with risk fac tors by multiple logistic regression analysis
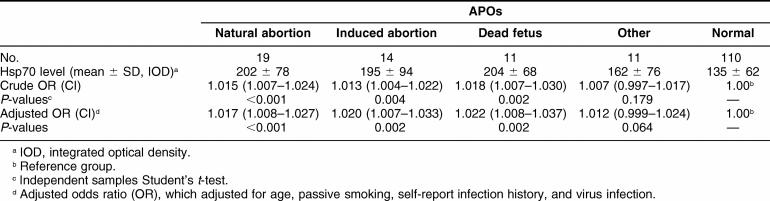
Table 4 presents lymphocyte Hsp70 levels by different types of APOs and their associations. As shown in this table, there were significantly positive correlations of increased Hsp70 with natural abortion (P < 0.001), induced abortion (P = 0.002), and dead fetus (P = 0.002). However, only borderline positive correlations of increased Hsp70 were observed in the combined group of other conditions, including 1 dead delivery, 4 preterm delivery, 5 abnormalities, and 1 intrauterine growth restriction (P = 0.064).
Table 4.
Lymphocyte Hsp70 levels in different types of adverse pregnancy outcomes (APOs) and their association with APOs
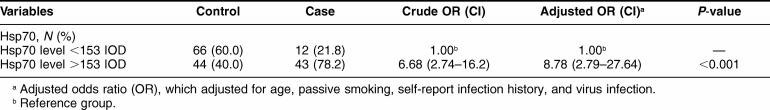
We then plotted a received operating characteristic (ROC) curve and established the cutoff value for IOD of Hsp70 levels and its predicted value of Hsp70 was 153. The sensitivity of the predicted value was 78%, the specificity was 60%, the positive predictive value (PPV) was 50%, and the negative predictive value (NPV) was 85% when this value (ie, Hsp70 = 153) was used to predict APOs among pregnant women. On the basis of this value, cases and controls were divided into 2 subgroups, and the numbers and percentage of each subgroup are listed in Table 5. This table also shows the crude and adjusted ORs and 95% CI for the associations of Hsp70 with APOs before (OR = 6.68, 95% CI = 2.74–16.28, P < 0.001) or after (OR = 8.78, 95% CI = 2.79–27.64, P < 0.001) adjustment for age, passive smoking, infection history, and virus infection in multivariate logistic regression analysis.
Table 5.
Logistic regression analysis of Hsp70 level in cases and controls
DISCUSSION
Human fetus development is a defined program that can be influenced by the plasticity of embryonic cells response to physiological and environmental changes within the maternal body through full pregnancy. The key period of this process is the first trimester of pregnancy for extraembryonic development and organogenesis. Many psychosocial, physiological, pathological, and environmental factors contribute to APOs, such as age, maternal stress, exposure to heavy metals, organic solvents, alcohol, ionizing radiation, and infection (Gardella and Hill 2000; Wadhwa et al 2001). It is very difficult, if not impossible, to fully investigate and understand these multiple and complicated risk factors of APOs. The features of the stress response (eg, low expression of Hsps under normal conditions and their fast overexpression after a wide range of stresses) and importance of Hsps (ie, cytoprotection, cellular differentiation, and mammalian embryonic development) suggested that these proteins could be used to evaluate the status of pregnancy. Human lymphocytes are often used as the common surrogate cells, and the microenvironment within the mother's body may influence fetal development and growth. However, the possible association of lymphocyte Hsp70 in early pregnant women with APOs is still unknown.
The results from this prospective nested case-control study showed that there was a significant increase in lymphocyte Hsp70 levels in early pregnant women with APOs compared with the well-matched controls. An age-related decrease in Hsp70 levels was observed as previously reported (Rao et al 1999; Jin et al 2004a). Our data showed that early pregnant women with lymphocyte Hsp70 levels >153 IOD have about 9-fold higher risk to develop APOs than those with lymphocyte Hsp70 levels <153 IOD, particularly in age-group 25–29. Therefore, age is a very important factor to investigate the role of Hsp70 as a biomarker in the evaluation of diseases and stress status.
It is well known that Hsps are expressed at low levels under normal and physiological conditions and that they rise to higher levels under stressful or pathological conditions. An unusual high Hsp70 level may indicate an overwhelming injury caused by pathological changes in the target tissues. For example, there are increased Hsp70 levels in lymphocytes of patients with heat-induced illness, even after recovery (Xiao et al 2003). Hsp70 levels in lymphocytes may represent a response to the continuous presence of harmful stimuli and/or an excess of damage that cannot be repaired as shown in the late stage of cerebral infarction (Jin et al 2004b). The cytoprotective role of Hsps is well documented for cultured cells, animals, transgenic animals, and humans. As indicated by our data, increased Hsp70 in lymphocytes of early pregnancy women may reflect a high level of stress in their early pregnancy in that APOs were more frequent in the group with higher levels of lymphocyte Hsp70.
Our data appear to be consistent with published data. For example, the results from Fukushima et al (2005) suggested that serum Hsp70 levels were particularly high in preterm delivery cases. In contrast, Child et al (2006) reported that there were nonsignificantly higher serum Hsp70 levels in 16-week gestational women who delivered babies with birth defects compared with well-matched controls and suggested that serum Hsp70 could be released from peripheral blood mononuclear cells (Hunter-Lavin et al 2004). However, the presence of antibodies against Hsp60 and Hsp70 in blood as well as Hsp60- and Hsp70-antibody complexes in the placenta has prognostic and diagnostic significance for subsequent birth outcomes (Shah et al 1998; Ziegert et al 1999). For example, antibodies against Hsp70 were significantly higher in women bearing fetus with birth defects (Child et al 2006). Indeed, our data showed an association between elevated lymphocyte Hsp70 levels and APOs. Further, it is reported that antibodies against Hsp70 are a marker of a prior increase in the levels of Hsp70 caused by stress that resulted in damage to the cells (Child et al 2006; Wu and Tanguay 2006). Unfortunately, we did not have data on antibodies against Hsp70 in the present study.
Furthermore, Hsps have also been shown to play a role in immunoreactions (Pockley 2003), in vitro fertilization and embryo transfer (Witkin et al 1994), and spontaneous abortion (Kligman et al 1998). The systematic analysis from Shah et al (1998) suggested that there were well-defined temporal and spatial patterns of Hsps expression in human placentas. Several investigations suggested that the expression of Hsps as a landmark of placenta stress response might have prognostic values (Ziegert et al 1999; Christians et al 2003). For example, one study found that the expression Hsp70 and Hsp90 increases in the chorionic villi of first trimester obtained from missed miscarriages compared to full-term placentas (Sotiriou et al 2004), and another study found that the expression of Hsp70 was also increased in tissues obtained from missed miscarriages compared with the controls (Hempstock et al 2003). However, the relationship between maternal lymphocyte Hsp70 and embryonic Hsp70 of early pregnant women remains unknown.
In the present study, we established the Hsp70 cutoff value at 153 IOD by the ROC curve. Our results showed that there seems to be a close correlation between Hsp70 and APOs: the pregnant women with elevated Hsp70 levels had a higher risk for APOs. The results also showed that the specificity was 60%, the sensitivity was 78%, the PPV was 50%, and the NPV was 85%. However, the level of Hsp70 alone was insufficient to predict APOs. Therefore, it is necessary to further investigate other possible predictors for APOs.
In summary, our present findings indicate that lymphocytic Hsp70 levels were associated with APOs. The mechanisms by which Hsp70 may play a role in the etiology of APOs and whether it can be applied as a clinical indicator of APOs warrant further investigation.
Acknowledgments
We are particularly grateful to all donors who volunteered to participate in this study and to members of the medical personnel of Hubei Hospital of Maternal Health Care for their generous help in the examination and sampling of subjects. This work was supported by research funds from the National Natural Science Foundation of China (30525031) and the National Key Basic Research and Development Program (2002CB512905) to T.W. T.W. and R.M.T. also acknowledge financial support from the NNSFC of China and the Canadian Institute of Health Research of Canada for a research exchange program and an operating CIHR grant (to R.M.T.).
REFERENCES
- Beck FX, Neuhofer W, Müller E. Molecular chaperones in the kidney: distribution, putative roles, and regulation. Am J Physiol Renal Physiol. 2000;279:F203–F215. doi: 10.1152/ajprenal.2000.279.2.F203.0363-6127(2000)279[F203:MCITKD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117.0009-7330(1998)083[0117:SHSPMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bonassi S, Au WW. Biomarkers in molecular epidemiology studies for health risk prediction. Mut Res. 2002;511:73–86. doi: 10.1016/s1383-5742(02)00003-0.0027-5107(2002)511[0073:BIMESF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9.0092-8674(1998)092[0351:THAHCM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Child DF, Hudson PR, Hunter-Lavin C, Mukhergee S, China S, Williams CP, Williams JH. Birth defect and anti-heat shock protein 70 antibodies in early pregnancy. Cell Stress Chaperones. 2006;11:101–105. doi: 10.1379/CSC-130R1.1.1466-1268(2006)011[0101:BDAASP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians ES, Zhou Q, Renard JP, Benjamin IJ. Heat shock proteins in mammalian development. Semin Cell Dev Biol. 2003;14:283–290. doi: 10.1016/j.semcdb.2003.09.021.1084-9521(2003)014[0283:HSPIMD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cogswell ME, Weisberg P, Spong C. Cigarette smoking, alcohol use and adverse pregnancy outcomes: implications for micronutrient supplementation. J Nutr. 2003;133:1722S–1731S.0022-3166(2003)133[1722S:CSAUAA]2.0.CO;2 [PubMed] [Google Scholar]
- Currie RW, Tanguay RM, Kingma JG. Heat-response and limitation of tissue necrosis during occusion/reperfusion in rabbit hearts. Circulation. 1993;87:963–971. doi: 10.1161/01.cir.87.3.963.0009-7322(1993)087[0963:HALOTN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Delmas F, Trocheris V, Murat JC. Expression of stress proteins in cultured HT29 human cell-line: a model for studying environmental aggression. Int J Biochem Cell Biol. 1995;27:385–391. doi: 10.1016/1357-2725(94)00069-n.1357-2725(1995)027[0385:EOSPIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fukushima A, Kawahara H, Isurugi C, Syoji T, Oyama R, Sugiyama T, Horiuchi S. Changes in serum levels of heat shock protein 70 in preterm delivery and pre-eclampsia. J Obstet Gynaecol Res. 2005;31:72–77. doi: 10.1111/j.1447-0756.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- Gardella JR, Hill JA III.. Environmental toxins associated with recurrent pregnancy loss. Semin Reprod Med. 2000;18:407–424. doi: 10.1055/s-2000-13731.0734-8630(2000)018[0407:ETAWRP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gibbs RS. The relationship between infections and adverse pregnancy outcomes: an overview. Ann Periodontol. 2001;6:153–163. doi: 10.1902/annals.2001.6.1.153. [DOI] [PubMed] [Google Scholar]
- Hempstock J, Jauniaux E, Greenwold N, Burton GJ. The contribution of placental oxidative stress to early pregnancy failure. Hum Pathol. 2003;34:1265–1275. doi: 10.1016/j.humpath.2003.08.006.0046-8177(2003)034[1265:TCOPOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2.0092-8674(1991)066[0191:HSSPCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075.0006-291X(2004)324[0511:HRFPBM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jin X, Wang R, and Xiao C. et al. 2004a Serum and lymphocyte levels of Hsp7l in aging: a study in the normal Chinese population. Cell Stress Chaperones. 9:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Xiao C, and Tanguay RM. et al. 2004b Correlation of lymphocyte Hsp71 levels with neurological deficits in elder patients with cerebral infarction. Am J Med. 117:406–411. [DOI] [PubMed] [Google Scholar]
- Kligman I, Jeremias J, Rosenwaks Z, Witkin SS. Cell-mediated immunity to human and Escherichia coli 60-kDa heat shock protein in women: association with a history of spontaneous abortion and endometriosis. Am J Reprod Immunol. 1998;40:32–36. doi: 10.1111/j.1600-0897.1998.tb00385.x.8755-8920(1998)040[0032:CITHAE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. J Nutr. 2003;133:1592S–1596S. doi: 10.1093/jn/133.5.1592S.0022-3166(2003)133[1592S:TEOAPO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Luft JC, Dix DJ. Hsp70 expression and function during embryogenesis. Cell Stress Chaperones. 1999;4:162–170. doi: 10.1379/1466-1268(1999)004<0162:heafde>2.3.co;2.1466-1268(1999)004[0162:HEAFDE]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metha TA, Greeman J, Ettelaie C, Venkatasubramaniam A, Chetter IC, McCollum PT. Heat shock proteins in vascular disease—a review. Eur J Endovasc Surg. 2005;29:395–402. doi: 10.1016/j.ejvs.2005.01.005.1078-5884(2005)029[0395:HSPIVD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Neuer A, Spandorfer SD, Giraldo P, Dieterle S, Rosenwaks Z, Witkin SS. The role of heat shock proteins in reproduction. Hum Reprod Update. 2000;6:149–159. doi: 10.1093/humupd/6.2.149.1355-4786(2000)006[0149:TROHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5.0140-6736(2003)362[0469:HSPARO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Plumier C, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997;2:162–167. doi: 10.1379/1466-1268(1997)002<0162:tmethi>2.3.co;2.1466-1268(1997)002[0162:TMETHI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao DV, Watson K, Jones GL. Age-related attenuation in the expression of the major heat shock proteins in human peripheral lymphocytes. Mech Ageing Dev. 1999;107:105–118. doi: 10.1016/s0047-6374(98)00143-2.0047-6374(1999)107[0105:AAITEO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shah M, Stanek J, Handwerger S. Differential localization of heat shock proteins 90, 70, 60 and 27 in human decidua and placenta during pregnancy. Histochem J. 1998;30:509–518. doi: 10.1023/a:1003259907014.0018-2214(1998)030[0509:DLOHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Patrick TE. Environmental exposures, toxicologic mechanisms, and adverse pregnancy outcomes. Am J Obstet Gynecol. 2005;192:S11–S21. doi: 10.1016/j.ajog.2004.06.117.1097-6868(2005)192[S11:EETMAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sotiriou S, Liatsos K, Ladopoulos I, Arvanitis DL. A comparison in concentration of heat shock proteins (HSP) 70 and 90 on chorionic villi of human placenta in normal pregnancies and in missed miscarriages. Clin Exp Obstet Gynecol. 2004;31:185–190.0390-6663(2004)031[0185:ACICOH]2.0.CO;2 [PubMed] [Google Scholar]
- Tanguay RM, Wu T 2006 Heat shock proteins in environmental stress and environment-related diseases. In: Heat Shock Proteins in Biology and Medicine, ed Radons J, Multhoff G. Research Signpost, Kerala, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguay RM, Wu Y, Khandjian EW. Tissue-specific expression of heat shock stress proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–118. doi: 10.1002/dvg.1020140205.1520-6408(1993)014[0112:TEOHSS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Culhane JF, Rauh V, Barve SS. Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J. 2001;5:119–125. doi: 10.1023/a:1011353216619. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063.0031-9333(1992)072[1063:MSRCPF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Witkin SS, Sultan KM, Neal GS, Jeremias J, Grifo JA, Rosenwaks Z. Unsuspected Chlamydia trachomatis infection and in vitro fertilization outcome. Am J Obstet Gynecol. 1994;171:1208–1214. doi: 10.1016/0002-9378(94)90134-1.1097-6868(1994)171[1208:UCTIAI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG. Elevated levels of circulating heat shock protein (Hsp70) in peripheral and renal vascular disease. Heart Vessels. 2000;15:18–22. doi: 10.1007/s003800070043.0910-8327(2000)015[0018:ELOCHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wu T, Tanguay RM. Antibodies against heat shock proteins in environmental stresses and diseases: friend or foe? Cell Stress Chaperones. 2006;11:1–12. doi: 10.1379/CSC-155R.1.1466-1268(2006)011[0001:AAHSPI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Tanguay RM, and Wu Y. et al. 1996 Presence of antibodies to heat stress proteins and its possible significance in workers exposed to high temperature and carbon monoxide. Biomed Environ Sci. 9:370–379. [PubMed] [Google Scholar]
- Xiao C, Chen S, and Li J. et al. 2002 Association of HSP70 and genotoxic damage in lymphocytes of workers exposed to coke-oven emission. Cell Stress Chaperones. 7:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Wu T, and Ren A. et al. 2003 Basal and inducible levels of Hsp70 in patients with acute heat illness induced during training. Cell Stress Chaperones. 8:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegert M, Witkin SS, Sziller I, Alexander H, Brylla E, Hartig W. Heat shock proteins and heat shock protein-antibody complexes in placental tissues. Infect Dis Obstet Gynecol. 1999;7:180–185. doi: 10.1002/(SICI)1098-0997(1999)7:4<180::AID-IDOG3>3.0.CO;2-7.1064-7449(1999)007[0180:HSPAHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]