Abstract
Heat shock protein (Hsp) 70 has been reported to protect various cells and tissues from ischemic damage. However, the molecular mechanisms of the protection are incompletely understood. Ischemia induces significant alterations in cellular redox status that plays a critical role in cell survival/death pathways. We investigated the effects of Hsp70 overexpression on cellular redox status in Madin-Darby canine kidney (MDCK) cells under both hypoxic and ischemic conditions with 3 different approaches: reactive oxygen species (ROS) measurement by a fluorescence probe, redox environment evaluation by a hydroxylamine spin probe, and redox status assessment by the glutathione/glutathione disulfide (GSH/GSSG) ratio. Results from each of these approaches showed that the redox status in Hsp70 cells was more reducing than that in control cells under either hypoxic or oxygen and glucose deprivation (OGD) conditions. In order to determine the mechanisms that mediated the alterations in redox state in Hsp70 cells, we measured the activities of glutathione peroxidase (GPx) and glutathione reductase (GR), two GSH-related antioxidant enzymes. We found that OGD exposure increased GPx and GR activities 47% and 55% from their basal levels (no stress) in Hsp70 cells, compared to only 18% and 0% increase in control cells, respectively. These data, for the first time, indicate that Hsp70 modulates the activities of GPx and GR that regulate cellular redox status in response to ischemic stress, which may be important in Hsp70's cytoprotective effects.
INTRODUCTION
Heat shock protein 70 (Hsp70) is a stress-responsive protein and is induced when cells are exposed to various types of stress including heat, hypoxia, and glucose deprivation. Increased levels of Hsp70 in turn result in a marked increase in stress tolerance and cytoprotection. Hsp70–mediated cytoprotective effects have been shown in many cell/tissue types under various environmental stresses and pathological conditions. Recently, many research groups have reported that Hsp70 is cytoprotective under ischemic conditions. For example, both in vitro and in vivo experiments have shown that induction of Hsp70 either by enforced overexpression with viral vectors or pharmacological approaches protects renal and brain cells from ischemic injuries (Papadopoulos et al 1996; Plumier et al 1997; Aufricht et al 1998; Bidmon et al 2000; Rajdev et al 2000; Lu et al 2002; Vicencio et al 2003; Yenari et al 2005). However, the molecular mechanisms that underlie the cytoprotective effects of Hsp70 in ischemic conditions are not fully understood.
Cellular redox environment, often termed redox status, refers to the reduction potential or reducing capacity in cellular or other biological systems (see Schafer and Buettner 2001 for review). Cellular redox status primarily is regulated by the balance between cellular oxidant and reductant levels. Cellular redox environment plays an important role in cellular functions, including regulation of proliferation, differentiation, and cell death (Schafer and Buettner 2001; Hiroi et al 2005). Many signaling molecules that play a critical role in cell death pathways are redox sensitive, such as cytochrome C, caspases, NF-κB, and AP-1, in that their levels and/or activations are controlled by cellular redox environment. For example, reactive oxygen species (ROS) can induce a series of specific cell death signaling events, including release of cytochrome C into the cytosol, activation of caspase-3, proteolytic cleavage of protein kinase C δ, and nuclear DNA fragmentation (Kajiwara et al 2001; Rejdak et al 2001; Anantharam et al 2002). Cellular redox status is altered in ischemia due to alterations in the levels and metabolism of oxygen and glucose (Shi and Liu 2006; Shi et al 2006). The critical role of elevated ROS levels in cell death caused by ischemia has provided the basis of a potential approach (antioxidant treatment) to minimize ischemic injury such as in renal tissues (Lloberas et al 2002).
Experimental evidence has suggested that there exists an interrelationship between Hsp70 and redox status. On one hand, both oxidative stress and antioxidants seem to regulate Hsp70 expression (Calabrese et al 2000a,b, 2001a; McLaughlin et al 2003). On the other hand, reduction in Hsp70 expression can increase ROS generation and mitochondrial protein oxidation (Yan et al 2002). Moreover, Hsp70 reduces cellular damage caused by oxidative stress in renal cells (Suzuki et al 2005). In contrast to these observations, the effect of Hsp70 on cellular redox status has not been evaluated directly, especially under ischemic conditions. We hypothesized that Hsp70 might protect cells from ischemic injuries by regulating cellular redox status. To test the hypothesis, we studied the redox status and cell death in Madin-Darby canine kidney (MDCK) cells overexpressed with Hsp70. Our results showed that Hsp70 improved cellular redox status in ischemic conditions. Furthermore, the results, for the first time, reported that Hsp70 significantly increased activities of glutathione peroxidase (GPx) and glutathione reductase (GR), which provides new insights into the mechanism of cytoprotection induced by Hsp70.
MATERIALS AND METHODS
Reagents
Dulbecco's modified Eagle's medium (DMEM), DMEM without glucose, newborn calf serum, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 2′,7′-dichlorofluorescin diacetate (DCFH-DA), trypsin, penicillin, and streptomycin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 1-Hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine (CMH) was from Alexis Biochem (Lausen, Switzerland). All other chemicals were of the highest grade available.
Cell culture and Hsp70 transfection
A construct containing the coding region for human Hsp70 (accession number NM_005345) was obtained from Dr. Anne Knowlton (University of California, Davis, CA, USA). The Hsp70 insert was amplified by polymerase chain reaction (PCR) and recloned into a pRC/CMV backbone. Parent MDCK cells were transfected with either a control pRC/CMV plasmid lacking an exogenous insert or with pRC/CMV-Hsp70 and incubated in medium containing 75 μg/mL G418 (Life Technologies, Inc., Grand Island, NY, USA). Individual colonies were isolated by limiting dilution and screened for expression of Hsp70 by Western blot analysis. A clone transfected with pRC/CMV that expressed control levels of Hsp70 protein (MDCK_pRC.1), as well as a clone transfected with pRC/ CMV-Hsp70 that expressed elevated levels of Hsp70 protein (MDCK_p70.2), were used in subsequent experiments.
Cell treatments
Cells were washed with warm phosphate-buffered saline (PBS) and the culture medium was changed to experimental medium (DMEM or DMEM without glucose). For hypoxic condition, DMEM medium was gassed previously with 95%N2/5%CO2 for 15 min. For oxygen and glucose deprivation (OGD), DMEM without glucose was gassed previously with 95%N2/5%CO2 for 15 minutes and was added to cell culture plates. Cells were incubated in a polymer hypoxic glove box (Coy Laboratory Products Inc., Grass Lake, MI, USA), in which the oxygen concentration was set at 1% and temperature at 37°C. For control experiments, cells were cultured in DMEM in a normoxic environment at 37°C.
Measurement of intracellular ROS
ROS levels were monitored using the cell-permeable probe DCFH-DA (Huang et al 2004). Cells were incubated with 100 μM DCFH-DA (dissolved in dimethyl sulfoxide [DMSO]) for 30 minutes at 37°C. After the incubation, cells were washed 3 times with PBS and the relative levels of fluorescence were quantified with a fluoromicroplate reader (excitation 485 nm and emission 535 nm). The measured fluorescence values were expressed as a percentage of the fluorescence in control cells.
Evaluation of redox status by electron paramagnetic resonance (EPR) spectroscopy
The oxidation of the cyclic hydroxylamine CMH was used to measure overall cellular redox state by EPR spectroscopy as described by Hwang et al 2003 and Menshikov et al 2006. Subconfluent cells were harvested with trypsin, washed once with PBS, and resuspended in the appropriate media (normal or OGD) at a concentration of 2 × 106 cells/mL. EPR samples were prepared by adding 100 μM CMH and 100 μM diethylenetriamine pentaacetate to the cell suspension. The cell mixture immediately was drawn into custom-made gas-permeable Teflon tubing (Zeus Industries, Raritan, NJ, USA), folded 4 times, and inserted into a quartz tube open at each end. EPR signal intensities were obtained at 5-minute intervals for 60 min. Oxygen tension (1%) in the cavity was achieved by perfusing a gas mixture of O2/N2/CO2 (1/94/5%). The oxygen tension reached balance in less than 5 min. Air was used for normoxic experiments. The EPR cavity temperature (37°C) was controlled by a Bruker Temperature Controller (Billerica, MA, USA). The EPR spectra were obtained with a Bruker EleXsys 540 x-band EPR spectrometer operating at 9.03 GHz and 100 kHz field modulation. Typical settings for the spectrometer were: magnetic field, 320 mT; scan range, 11 mT; microwave power, 10 mW; modulation amplitude, 0.1 mT; time constant, 0.16 s. The EPR spectra were collected, stored, and manipulated using the Bruker Software Xepr®.
Measurement of glutathione/glutathione disulfide ratio
A widely used indicator of cellular redox environment (status; glutathione [GSH] to glutathione disulfide [GSSG] ratio) (Schafer and Buettner 2001) also was used to determine cellular redox status in Hsp70 cells under various exposures. The ratio was measured with a kit from Cayman (kit 703002, Ann Arbor, MI, USA), which employs a spectrophotometeric recycling assay (Lee et al 1998; Dringen et al 1999). Briefly, cells were scraped into cold PBS and centrifuged. The cell pellets were frozen at −80°C. Cells were thawed and homogenized in a cold buffer containing 0.2 M 2-(N-morpholino) ethanesulphonic acid, 50 mM phosphate, 1 mM EDTA, pH 6.0, and centrifuged at 10 000 g for 15 minutes at 4°C. The supernatants were removed for analyses according to the manufacturer's instruction. All the determinations were normalized to protein content determined by the method of Lowry et al (1951). The absorbances were recorded at 405 nm using a plate reader at 5-minute intervals for 30 min.
Measurement of GPx activity
GPx activity was measured by using a glutathione peroxidase assay kit (Cayman). Briefly, cells were scraped, collected, and homogenized in cold 50 mM potassium phosphate (pH 7.5, 1 mM EDTA). After the homogenates were centrifuged at 10 000 g for 15 minutes at 4°C, the supernatants were removed for analyses. The samples containing 50 μg total proteins were added to a solution containing 1 mM GSH, 0.4 unit/mL glutathione reductase, and 0.2 mM nicotinamide adenine dinucleotide phosphate (NADPH). The reaction was initiated by adding substrate cumene hydroperoxide (final concentration: 0.22 mM), and the reduction was recorded at 340 nm using a kinetic program (6 readings at 1-minute intervals). The GPx activity was determined by the rate of decrease in absorbance at 340 nm (1 mU/mL GPx = [A340/min]/ 0.0062). Results were normalized by total protein.
Measurement of GR activity
The activity of GR was detected using a glutathione reductase assay kit (Cayman) according to the manufacturer's instruction. Briefly, cells were scraped, collected, and homogenized in cold 50 mM potassium phosphate (pH 7.5, 1 mM EDTA). After the homogenates were centrifuged at 10 000 g for 15 minutes at 4°C, the supernatants were removed for assay. The activity of GR was calculated using the following formula: GR activity = (ΔA340/ min)/0.00373 μM−1 × 9.5 × dilution factor. One unit was defined as the amount of enzyme that caused the oxidation of 1.0 nmol of NADPH to NADP+/minute at 25°C.
Assessment of cell viability by 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) method
Cells (10 000 cells/well) cultured in 96-well NUNC plates (Nalge Nunc, NY, USA) were treated under hypoxia or OGD condition for 3 hours. Cell viability was measured 24 hours after the hypoxic and OGD treatments. The number of viable cells was determined by CellTiter 96AQ One Solution Cell Proliferation Assay (Promega, Madison, WI, USA). The absorbance at 490 nm was measured using a Bio-Rad 3350 microplate reader.
Measurement of cell death by lactate dehydrogenase release assay
The release of lactate dehydrogenase (LDH) from cells was used to detect cytotoxicity in cells exposed to the different experimental conditions. Briefly, the cell-free culture medium (100 μL) was collected and then incubated with 100 μL of the reaction mixture from the Cytotoxicity Detection Kit (Takara Bio Inc., Shiga, Japan) for 30 minutes at room temperature. The optical density of the solution was then measured at 490 nm (655 nm as reference) on a Bio-Rad 3350 microplate reader. Extract from cells lysed with 1% Triton X-100 was used as 100% cell death.
Statistical analysis
One-way ANOVA was used to estimate overall significance followed by post hoc Tukey's tests corrected for multiple comparisons. Data are presented as mean ± standard error of mean (SEM), if not otherwise noted. Difference was considered significant at P < 0.05.
RESULTS
Hsp70 levels in Hsp70 and control cells
The relative expression of Hsp70 protein in the cell lines used in these studies is shown in Figure 1. Hsp70 protein was detected as a very light band in the cells expressing the control empty plasmid by Western blotting. In contrast, abundant levels of protein were measured in the MDCK cells transfected with the Hsp70 expression vector.
Fig 1.
Expression of heat shock protein (Hsp) 70 in transfected cells. Hsp70 protein expression was measured by a Western blot in either control MDCK_pRC.1 cells (polymerase chain reaction [PRC]) or in MDCK_p70.2 cells (Hsp70)
Effects of Hsp70 on intracellular ROS levels
Cellular redox status is determined by the overall cellular oxidant and reductant levels. The level of cellular ROS is one of the markers to indicate cellular redox status. The higher the ROS level, the more oxidizing the cellular redox environment. We examined the intracellular ROS levels in Hsp70 and control cells after in vitro hypoxic and ischemic treatments by the cell-permeable probe DCFH-DA. As shown in Figure 2, both hypoxic and OGD exposures led to increases in DCF fluorescence in control and Hsp70 cells, which reflected increases in cellular ROS levels. Hsp70 suppressed the ROS level induced by hypoxia (13% increase in Hsp70 cells vs 51% in control cells, P < 0.01). The increase in ROS levels in Hsp70 cells (52%) was significantly less than that in control cells (82%) after OGD treatment. These results indicate that the overexpression of Hsp70 repressed ROS levels when cells were exposed to either hypoxia or OGD.
Fig 2.
Effects of heat shock protein (Hsp) 70 on intracellular reactive oxygen species reactive oxygen species (ROS) levels after hypoxia and oxygen and glucose deprivation (OGD) treatments. Hsp70 transfected cells (Hsp70) and control cells (polymerase chain reaction [PRC]) were treated under normal condition (21% O2) (N), hypoxic condition (1% O2) (H), and OGD for 3 h. Data are the mean ± standard error of mean. 3 sets of experiments were carried out at different days with 3 repeats per set. The results were normalized by protein content. a, P < 0.05; b, P < 0.01 vs normal condition; c, P < 0.05; d, P < 0.01 vs hypoxic condition; e, P < 0.05; f, P < 0.01 vs PRC cells under the same treatment
Effects of Hsp70 on cellular redox status detected by EPR techniques
CMH is a hydroxylamine, which is EPR “silent”, but can be oxidized to a nitroxide, which is EPR “active”. The nitroxide can be reduced back to CMH in a more reducing environment. Thus, EPR measurements of oxidized CMH can be used as a probe to report the redox status in cells. Figure 3A shows an EPR spectrum when CMH was incubated with PRC cells under OGD condition, indicating that CMH was oxidized efficiently over the period of observation. The rate of the increase in the EPR signal then was used to measure the cellular redox status. A higher rate indicates a more oxidizing environment and a lower rate indicates a more reducing environment. By monitoring the heights of EPR signal peaks at low field of the spectra, we obtained the oxidation rate of CMH as shown in Figure 3B. The rates in control and Hsp70 cells during their exposures to hypoxia and OGD are shown in Table 1. Under normoxic condition, the rates of CMH oxidation in the 2 cell types were similar. Both hypoxic and OGD exposures increased the rates in the 2 cell types. The increase in Hsp70 cells was significantly lower than that in control cells, especially under OGD exposure. These results suggest that Hsp70 cells had more reducing power to keep the oxidation of CMH lower than that control cells during both hypoxia and OGD exposures, which was in accordance to our observations on cellular ROS levels.
Fig 3.
Electron paramagnetic resonance (EPR) detection of redox status with 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine (CMH) as a redox probe. The oxidation of the cyclic hydroxylamine CMH (100 μM) in heat shock protein (Hsp) 70 transfected cells and control cells was monitored by x-band EPR spectroscopy in a real time manner. (A) A representative EPR spectrum of CMH oxidation product, a nitroxide. (B) Representatives of the time-dependent oxidation of CMH in cells. The rate was a parameter to indicate cellular redox status. The higher the rate, the more oxidizing the cells; the lower the rate, the more reducing the cells. See Table 1 for the effect of Hsp70 on the oxidation rate of CMH in cells exposed to different treatments
Table 1.
Effects of heat shock protein (Hsp) 70 on cellular redox status measured by 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetra \[chmethyl-pyrrolidine (CMH) oxidation
Effects of Hsp70 on cellular redox status GSH/GSSG ratio
The ratio of GSH/GSSG has been widely used to indicate cellular redox status (Schafer and Buettner 2001). It has been reported that OGD exposure causes oxidative stress and a decreased GSH/GSSG ratio (Jiang et al 2004; Shi and Liu 2006). We conducted experiments to measure levels of GSH and GSSG and the GSH/GSSG ratio to further understand the redox status in Hsp70 cells when they were exposed to ischemic stress. The absolute values of cellular GSH and GSSG levels in the 2 cell lines after hypoxic and OGD exposures are listed in Table 2. As shown in Figure 4, hypoxic exposure reduced the GSH/ GSSG ratio to 79.5% in control cells and 87.7% in Hsp70 cells. OGD treatment decreased the GSH/GSSG ratio to 59.1% in control cells and 76.1% in Hsp70 cells (P < 0.01). These results, which are in agreement with the observations on using CMH as a redox probe, indicate that Hsp70 can improve the redox environments when cells are exposed to stresses such as hypoxia and OGD.
Table 2.
Effects of heat shock protein (Hsp) 70 on glutathione (GSH) and glutathione disulfide (GSSG) levels under hypoxic and oxygen and glucose deprivation (OGD) exposures
Fig 4.
Effects of heat shock protein (Hsp) 70 on cellular redox status (glutathione/glutathione disulfide [GSH/GSSG] ratio) after hypoxia and oxygen and glucose deprivation (OGD) treatments. Hsp70 transfected cells (Hsp70) and control cells (polymerase chain reaction [PRC]) were treated under normal condition (21% O2) (N), hypoxic condition (1% O2) (H), and OGD for 3 h. Data are expressed as mean ± standard error of mean. 3 sets of experiments were carried out at different days with 3 repeats per set. a, P < 0.05; b, P < 0.01 vs normal condition; c, P < 0.05; d, P < 0.01 vs hypoxic condition; e, P < 0.01 vs PRC cells under the same treatment. See Table 2 for the absolute values of GSH and GSSG levels in cells exposed to different treatments
Effects of Hsp70 on activities of GSH-related antioxidant enzymes
GSH is a critical small molecular antioxidant and plays a critical role in reducing free radicals and maintaining cellular redox status in cells. GPx and GR are 2 enzymes that assist GSH in scavenging oxidants and stabilizing the intracellular redox status. The increased reducing activity in Hsp70 cells exposed to an OGD stress prompted us to investigate the activities of GPx and GR under the experimental conditions. As shown in Figure 5, hypoxic exposure enhanced the activity of GPx to 59.9 nmol/min/ ml (10% increase) in control cells and 31.1 nmol/min/ml (9% increase) in Hsp70 cells. Remarkably, OGD treatment increased the activity of GPx from 54.3 to 64.3 nmol/ min/ml in control cells (18% increase), and from 28.7 to 42.0 nmol/min/ml in Hsp70 cells (47% increase; P < 0.01). Figure 6 shows that the activity of GR was increased 18% in Hsp70 cells and 11% in control cells under hypoxic exposure. OGD treatment significantly increased 55% of the activity of GR in Hsp70 cells, although no increase was observed in control cells (P < 0.01). These results are evident that Hsp70 cells had a high ability to increase the 2 enzymes' activities in response to hypoxic and OGD exposures. Moreover, we observed that, under normal condition, the activities of GPx and GR in Hsp70 cells were lower than those in control cells (control vs Hsp70 cells: GPx, 54.3 vs 28.7; GR, 17.2 vs 10.7) (Figs 5A, 6A), indicating that Hsp70 cells may need lower activities of the antioxidant enzymes when cells are not exposed to stress.
Fig 5.
Effects of heat shock protein (Hsp) 70 on glutathione peroxidase (GPx) activity after hypoxia and oxygen and glucose deprivation (OGD) treatments. Hsp70 transfected cells (Hsp70) and control cells (polymerase chain reaction [PRC]) were treated under normal condition (21% O2) (N), hypoxic condition (1% O2) (H), and OGD for 3 h. Data are expressed as mean ± standard error of mean. 3 sets of experiments were carried out at different days with 3 repeats per set. (A) GPx activity; (B) ratio between different treatments to base levels (normal condition). a, P < 0.05; b, P < 0.01 vs normal condition; c, P < 0.01 vs hypoxic condition; d, P < 0.01 vs PRC cells under the same treatment
Fig 6.
Effects of heat shock protein (Hsp) 70 on glutathione reductase (GR) activity after hypoxia and oxygen and glucose deprivation (OGD) treatments. Hsp70 transfected cells (Hsp70) and control cells (polymerase chain reaction [PRC]) were treated under normal condition (21% O2) (N), hypoxic condition (1% O2) (H), and OGD for 3 h. Data are expressed as mean ± standard error of mean. 3 sets of experiments were carried out at different days with 3 repeats per set. (A) GR activity; (B) ratio between different treatments to base levels (normal condition). a, P < 0.01 vs normal condition; b, P < 0.01 vs hypoxic condition; c, P < 0.01 vs PRC cells under the same treatment
Effect of Hsp70 overexpression on cytotoxicity induced by hypoxia and OGD
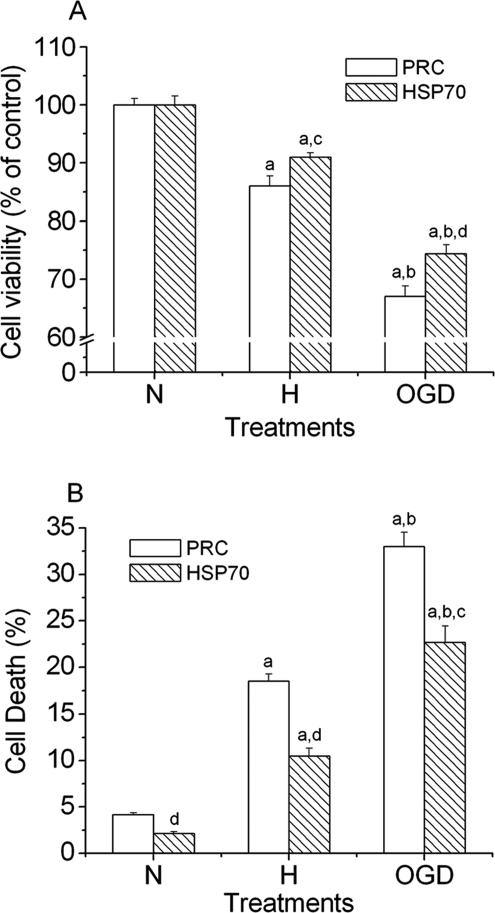
To assess the effect of overexpression of Hsp70 on cytotoxicity induced by hypoxic and OGD treatments, we used 2 common methods, MTT cell proliferation assay and lactate dehydrogenase (LDH) release assay, to measure the cell viability. As shown in Figure 7A (MTT assay), cell viability decreased to 86.1% in control cells and 91.0% in Hsp70 cells (P < 0.05 vs control) in the hypoxic experiments, and the cell viability decreased to 67.0% in control cells and 74.4% in Hsp70 cells (P < 0.01 vs control) in the OGD experiments. Similarly, the results from LDH assay showed that hypoxic treatment caused 18.5% (control cells) and 10.5% (Hsp70 cells) cell death (Fig 7B). OGD treatment increased cell death to 33.0% (control cells) and 22.7% (Hsp70 cells). These results suggested that cell death caused by hypoxia and OGD was approximately 50% and 30%, respectively, less in Hsp70 cells than in control cells. These observations provide evidence that Hsp70 was cytoprotective under either hypoxic or OGD conditions.
Fig 7.
Effects of heat shock protein (Hsp) 70 on cell viability after hypoxia and oxygen and glucose deprivation (OGD) treatments. Hsp70 transfected cells (Hsp70) and control cells (polymerase chain reaction [PRC]) were incubated under normal condition (21% O2) (N), hypoxic condition (1% O2) (H), and OGD for 3 h, and then subjected to 24-h culture under normal condition. Cell death was assessed by both 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) (A) and lactate dehydrogenase (LDH) release (B) assays. Data are expressed as mean ± standard errror of the mean, n = 6. a, P < 0.01 vs normal condition; b, P < 0.01 vs hypoxic condition; c, P < 0.05; d, P < 0.01 vs PRC cells under the same treatment
DISCUSSION
Our results show for the first time that overexpression of Hsp70 maintains a more reducing intracellular environment during insult by hypoxia or ischemia. Furthermore, we demonstrate that the activities of 2 antioxidative enzymes, GPx and GR, is enhanced markedly in cells overexpressing Hsp70 compared to that in control cells in response to ischemic stress. The results suggest that maintaining cellular redox environment through increased functions of these enzymes accounts, at least in part, for Hsp70–mediated protective effects in cells exposed to hypoxia/ischemia.
In counteracting the constant generation of oxidants during normal metabolism, cells have developed a network of defense systems, mediated by small molecule antioxidants and enzymes. Among these antioxidants, GSH is considered vital for cell survival (Cooper and Kristal 1997; Li et al 1997; Almeida et al 2002). Cellular damage associated with oxidative stress has been reported following GSH depletion (Papadopoulos et al 1997; Gupta et al 2000; Mytilineou et al 2002; Vexler et al 2003). GSH functions as a key player in the intracellular redox regulation (Gabbita et al 2000) because of its high reduction potential and high intracellular concentrations (5–10 mM in many cells). It maintains the reduced state of the cysteinyl-thiol groups of proteins and rescues cells from apoptosis by buffering an endogenously induced oxidative stress (Filomeni et al 2002; Schulz et al 2000). GSH also acts as a coenzyme of many enzymes involved in cell defense, such as GPx, which utilizes GSH to detoxify H2O2, an inducer of cell death involving both necrosis and apoptosis (Saito et al 2006). GSH transforms to GSSG after reacting with oxidants, and recycling GSSG is crucial to supply GSH in cells, especially under oxidative stress. GR is responsible for reducing GSSG back to reutilizable GSH in cells (Meister and Anderson 1983). Thus, through the activities of both GPx and GR, GSH effectively scavenges oxidants such as H2O2 and maintains the cellular redox environment. The responses of the Hsp70 cells to OGD exposure are notable in terms of the increase in the activities of GPx and GR. GPx activity increased 47% from its base level in Hsp70 cells, compared to 18% in control cells after 3 hours OGD exposure. Similarly, the activity of GR was enhanced remarkably in Hsp70 cells in response to the exposure. These results suggest that GPx and GR are 2 enzymes that are highly modulated by Hsp70 in response in vitro ischemic exposure. Accordingly, we observed decreased levels of ROS and higher GSH/GSSG ratio in Hsp70 transfected cells, compared to control cells, when exposed to OGD. In agreement to these observations, a previous report has suggested a cytoprotective effect of Hsp70 following oxidative stress through a mechanism that was proposed to be mediated by elevations in glutathione levels (Xu and Giffard 1997).
It is interesting that the Hsp70 overexpressed cells maintain a quite low level of GPx and GR activity under normal condition though they have a higher inducing rate under OGD exposure but not the hypoxic condition. This observation may be attributed to the roles of glucose. Glucose not only provides energy but also sustains a cellular reducing environment by generating reducing agents, such as NADPH, through the pentose phosphate pathway (Kletzien et al 1994; Delgado-Esteban et al 2000; Almeida et al 2002). This pathway has been suggested to be the major source of NADPH production for the maintenance of cellular GSH, which is critical in maintaining the cellular milieu in a reduced state (Ben-Yoseph et al 1994; Delgado-Esteban et al 2000; Almeida et al 2002). Under hypoxia (in the presence of glucose), cellular redox status may not be altered significantly, compared to that under OGD exposure, where deprivation of glucose alters cellular redox status significantly. The results indicate that Hsp70 protein can sense the severity of the stress and respond to distinct stresses differently.
One critical and well-studied role of Hsp70 is its chaperone activity. It has been suggested that Hsps may bind virtually every protein made within the cell. Hsps may even actively participate in cellular protein regulations under normal (nonstress) condition. Our observation that GPx and GR activities were lower in Hsp70 cells than in control cells under normal condition is in line with this concept. It may indicate that Hsp70 regulates the enzyme activities to match cells' needs in different conditions. As a chaperone, the interaction between Hsp70 and its target proteins may be important in maintaining a functional protein in the following possible mechanisms: reducing abnormal protein folding during translation, assisting protein to fold into its functional conformation, transporting proteins to certain intracellular locations, assembling protein complexes, and even regulating the availability of a receptor or activity of an enzyme (Beckmann et al 1990; Moseley 1998; Soti and Csermely 2006). It is clear from our results that Hsp70 significantly promotes the activities of 2 GSH-related enzymes, GPx and GR, in response to OGD stress (Figs 5, 6). Thus, regulating the proteins' functions may be responsible for the effect of Hsp70 on cell death. The mechanisms by which Hsp70 enhances the induction of GPx and GR after stress and, importantly, whether or not the increase in the activities is related to its chaperone activity are not clear, although currently a focus of experimental activity in our laboratories.
Besides its role as a molecular chaperone, it has been suggested that Hsp70 may interfere with cell death pathways by directly regulating the expression and/or activation of proapoptotic and antiapoptoic proteins. It has been reported that Hsp70 acts at several stages of the apoptotic pathway. Hsp70 inhibits the release of cytochrome c from mitochondria and hence acts upstream of the formation of the apoptosome (Steel et al 2004). It also sequesters apoptosis inducing factor (Gurbuxani et al 2003) and decreases the cleavage of the common death substrate protein poly(ADP-ribose) polymerase (Mosser et al 1997). It increases the expression of the antiapoptotic protein Bcl-2 after ischemia (Kelly et al 2002; Yenari et al 2005). In addition, it has been suggested that Hsp70 exerts protective effects through an inhibition of NF-κB signaling pathway (Calabrese et al 2001b). A resolution of these divergent theories of the role of Hsp70 in the response of cells to various stresses is critical to a full understanding of the molecular events underlying Hsp70 activities. However, the mechanism of Hsp70 in preventing apoptosis is still not fully understood. As cellular redox status ubiquitously regulates pathways of apoptosis, including the steps described above, Hsp70′s ability to regulate the redox environment of a cell might well prove to be a central and unifying event in its cytoprotection. As observed in this study, 2 important redox enzymes are modulated by Hsp70 in cells exposed to stress. The improved redox environment is likely responsible for the ability of Hsp70 to reduce cell injury caused by ischemic insult. Nevertheless, the two enzymes may be two examples that Hsp70 regulates in cells to counteract harmful effects induced by stress. For example, it has been found that Hsp70 may bind to and modulate the function of BAG-1, the bcl-2 binding protein, thus modulating some types of apoptosis-related cell death (McLaughlin et al 2003).
In conclusion, our results show that Hsp70 can regulate cellular redox status through modulating the activities of the GSH-related enzymes, GPx and GR, in response to hypoxic and ischemic stress. The modulation of antioxidant enzyme activities may be a potential critical mechanism that mediates the enhanced cytoprotection afforded by enhanced expression of Hsp70.
Acknowledgments
We thank the technical help provided by R. Purvis at College of Pharmacy, University of New Mexico. This research was supported in part by grants from National Institutes of Health (P20 RR15636, AR40771, P30-012072) and American Heart Association (0565508Z).
REFERENCES
- Almeida A, Delgado-Esteban M, Bolanos JP, Medina JM. Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. J Neurochem. 2002;81:207–217. doi: 10.1046/j.1471-4159.2002.00827.x.0022-3042(2002)081[0207:OAGDIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3–dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress–mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22:1738–1751. doi: 10.1523/JNEUROSCI.22-05-01738.2002.0270-6474(2002)022[1738:CPCOPK]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufricht C, Lu E, Thulin G, Kashgarian M, Siegel NJ, Van Why SK. ATP releases Hsp-72 from protein aggregates after renal ischemia. Am J Physiol. 1998;274:F268–274. doi: 10.1152/ajprenal.1998.274.2.F268.0002-9513(1998)274[F268:ARHFPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. doi: 10.1126/science.2188360.0193-4511(1990)248[0850:IOHWNS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph O, Boxer PA, Ross BD. Oxidative stress in the central nervous system: monitoring the metabolic response using the pentose phosphate pathway. Dev Neurosci. 1994;16:328–336. doi: 10.1159/000112127.0378-5866(1994)016[0328:OSITCN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bidmon B, Endemann M, Muller T, Arbeiter K, Herkner K, Aufricht C. Heat shock protein 70 repairs proximal tubule structure after renal ischemia. Kidney Int. 2000;58:2400–2407. doi: 10.1046/j.1523-1755.2000.00423.x.0085-2538(2000)058[2400:HSPRPT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Calabrese V, Copani A, and Testa D. et al. 2000a Nitric oxide synthase induction in astroglial cell cultures: effect on heat shock protein 70 synthesis and oxidant/antioxidant balance. J Neurosci Res. 60:613–622. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Catalano C, Bates TE, Dinotta F, Micali G, Giuffrida Stella AM. Induction of heat shock protein synthesis in human skin fibroblasts in response to oxidative stress: regulation by a natural antioxidant from rosemary extract. Int J Tissue React. 2001a;23:51–58.0250-0868(2001)023[0051:IOHSPS]2.0.CO;2 [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Giuffrida Stella AM, Bates TE, Clark JB. Mitochondrial involvement in brain function and dysfunction: relevance to aging, neurodegenerative disorders and longevity. Neurochem Res. 2001b;26:739–764. doi: 10.1023/a:1010955807739.0364-3190(2001)026[0739:MIIBFA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Calabrese V, Testa G, Ravagna A, Bates TE, Stella AM. Hsp70 induction in the brain following ethanol administration in the rat: regulation by glutathione redox state. Biochem Biophys Res Commun. 2000b;269:397–400. doi: 10.1006/bbrc.2000.2311.0006-291X(2000)269[0397:HIITBF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Kristal BS. Multiple roles of glutathione in the central nervous system. Biol Chem. 1997;378:793–802.1431-6730(1997)378[0793:MROGIT]2.0.CO;2 [PubMed] [Google Scholar]
- Delgado-Esteban M, Almeida A, Bolanos JP. D-Glucose prevents glutathione oxidation and mitochondrial damage after glutamate receptor stimulation in rat cortical primary neurons. J Neurochem. 2000;75:1618–1624. doi: 10.1046/j.1471-4159.2000.0751618.x.0022-3042(2000)075[1618:DPGOAM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999.0270-6474(1999)019[0562:SOTAGI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filomeni G, Rotilio G, Ciriolo MR. Cell signalling and the glutathione redox system. Biochem Pharmacol. 2002;64:1057–1064. doi: 10.1016/s0006-2952(02)01176-0.0006-2952(2002)064[1057:CSATGR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gabbita SP, Robinson KA, Stewart CA, Floyd RA, Hensley K. Redox regulatory mechanisms of cellular signal transduction. Arch Biochem Biophys. 2000;376:1–13. doi: 10.1006/abbi.1999.1685.0003-9861(2000)376[0001:RRMOCS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gupta A, Datta M, Shukla GS. Cerebral antioxidant status and free radical generation following glutathione depletion and subsequent recovery. Mol Cell Biochem. 2000;209:55–61. doi: 10.1023/a:1007000430394.0300-8177(2000)209[0055:CASAFR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gurbuxani S, Schmitt E, and Cande C. et al. 2003 Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene. 22:6669–6678. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Ogihara T, Hirano K, Hasegawa M, Morinobu T, Tamai H, Niki E. Regulation of apoptosis by glutathione redox state in PC12 cells exposed simultaneously to iron and ascorbic acid. Free Radic Biol Med. 2005;38:1057–1072. doi: 10.1016/j.freeradbiomed.2005.01.001.0891-5849(2005)038[1057:ROABGR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Huang HM, Zhang H, Ou HC, Chen HL, Gibson GE. Alpha-keto-beta-methyl-n-valeric acid diminishes reactive oxygen species and alters endoplasmic reticulum Ca(2+) stores. Free Radic Biol Med. 2004;37:1779–1789. doi: 10.1016/j.freeradbiomed.2004.08.001.0891-5849(2004)037[1779:AADROS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hwang J, Saha A, and Boo YC. et al. 2003 Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 278:47291–47298. [DOI] [PubMed] [Google Scholar]
- Jiang X, Mu D, and Manabat C. et al. 2004 Differential vulnerability of immature murine neurons to oxygen-glucose deprivation. Exp Neurol. 190:224–232. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Ikeda K, Kuroi R, Hashimoto R, Tokumaru S, Kojo S. Hydrogen peroxide and hydroxyl radical involvement in the activation of caspase-3 in chemically induced apoptosis of HL-60 cells. Cell Mol Life Sci. 2001;58:485–491. doi: 10.1007/PL00000872.1420-682X(2001)058[0485:HPAHRI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Zhang ZJ, and Zhao H. et al. 2002 Gene transfer of Hsp72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: influence of Bcl-2. Ann Neurol. 52:160–167. [DOI] [PubMed] [Google Scholar]
- Kletzien RF, Harris PK, Foellmi LA. Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J. 1994;8:174–181. doi: 10.1096/fasebj.8.2.8119488.0892-6638(1994)008[0174:GDAHES]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Galoforo SS, and Berns CM. et al. 1998 Glucose deprivation– induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J Biol Chem. 273:5294–5299. [DOI] [PubMed] [Google Scholar]
- Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8.0896-6273(1997)019[0453:ARFLIN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lloberas N, Torras J, Herrero-Fresneda I, Cruzado JM, Riera M, Hurtado I, Grinyo JM. Postischemic renal oxidative stress induces inflammatory response through PAF and oxidized phospholipids. Prevention by antioxidant treatment. FASEB J. 2002;16:908–910. doi: 10.1096/fj.01-0880fje.0892-6638(2002)016[0908:PROSII]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.0021-9258(1951)193[0265:PMWTFP]2.0.CO;2 [PubMed] [Google Scholar]
- Lu A, Ran R, Parmentier-Batteur S, Nee A, Sharp FR. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem. 2002;81:355–364. doi: 10.1046/j.1471-4159.2002.00835.x.0022-3042(2002)081[0355:GIHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- McLaughlin B, Hartnett KA, Erhardt JA, Legos JJ, White RF, Barone FC, Aizenman E. Caspase 3 activation is essential for neuroprotection in preconditioning. Proc Natl Acad Sci U S A. 2003;100:715–720. doi: 10.1073/pnas.0232966100.1091-6490(2003)100[0715:CAIEFN]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A, Anderson ME. Glutathione. Ann Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431.0066-4154(1983)052[0711:G]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Menshikov M, Plekhanova O, and Cai H. et al. 2006 Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arterioscler Thromb Vasc Biol. 26:801–807. [DOI] [PubMed] [Google Scholar]
- Moseley PL. Heat shock proteins and the inflammatory response. Ann N Y Acad Sci. 1998;856:206–213. doi: 10.1111/j.1749-6632.1998.tb08327.x.0077-8923(1998)856[0206:HSPATI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317.1098-5549(1997)017[5317:ROTHHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mytilineou C, Kramer BC, Yabut JA. Glutathione depletion and oxidative stress. Parkinsonism Relat Disord. 2002;8:385–387. doi: 10.1016/s1353-8020(02)00018-4.1353-8020(2002)008[0385:GDAOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Koumenis IL, Dugan LL, Giffard RG. Vulnerability to glucose deprivation injury correlates with glutathione levels in astrocytes. Brain Res. 1997;748:151–156. doi: 10.1016/s0006-8993(96)01293-0.0006-8993(1997)748[0151:VTGDIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Sun XY, Cao J, Mivechi NF, Giffard RG. Overexpression of Hsp-70 protects astrocytes from combined oxygen-glucose deprivation. Neuroreport. 1996;7:429–432. doi: 10.1097/00001756-199601310-00013.0959-4965(1996)007[0429:OOHPAF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Plumier JC, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997;2:162–167. doi: 10.1379/1466-1268(1997)002<0162:tmethi>2.3.co;2.1466-1268(1997)002[0162:TMETHI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–791.0364-5134(2000)047[0782:MORHSP]2.0.CO;2 [PubMed] [Google Scholar]
- Rejdak R, Rejdak K, Sieklucka-Dziuba M, Stelmasiak Z, Grieb P. Brain tolerance and preconditioning. Pol J Pharmacol. 2001;53:73–79.1230-6002(2001)053[0073:BTAP]2.0.CO;2 [PubMed] [Google Scholar]
- Saito Y, Nishio K, and Ogawa Y. et al. 2006 Turning point in apoptosis/ necrosis induced by hydrogen peroxide. Free Radic Res. 40:619–630. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/ glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4.0891-5849(2001)030[1191:REOTCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress, and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x.0014-2956(2000)267[4904:GOSAN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shi H, Liu K. Effects of glucose concentration on redox status in rat primary cortical neurons under hypoxia. Neurosci Lett. 2006;410:57–61. doi: 10.1016/j.neulet.2006.09.066.0304-3940(2006)410[0057:EOGCOR]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Liu S, Miyake M, Liu KJ. Ebselen induced C6 glioma cell death in oxygen and glucose deprivation. Chem Res Toxicol. 2006;19:655–660. doi: 10.1021/tx0502544.0893-228X(2006)019[0655:EICGCD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soti C, Csermely P 2006 Pharmacological modulation of the heat shock response. Handb Exp Pharmacol: 417–436. [DOI] [PubMed] [Google Scholar]
- Steel R, Doherty JP, Buzzard K, Clemons N, Hawkins CJ, Anderson RL. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem. 2004;279:51490–51499. doi: 10.1074/jbc.M401314200.0021-9258(2004)279[51490:HIAUOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Maruyama S, and Sato W. et al. 2005 Geranylgeranylacetone ameliorates ischemic acute renal failure via induction of Hsp70. Kidney Int. 67:2210–2220. [DOI] [PubMed] [Google Scholar]
- Vexler ZS, Wong A, and Francisco C. et al. 2003 Fructose-1,6-bisphosphate preserves intracellular glutathione and protects cortical neurons against oxidative stress. Brain Res. 960:90–98. [DOI] [PubMed] [Google Scholar]
- Vicencio A, Bidmon B, and Ryu J. et al. 2003 Developmental expression of Hsp72 and ischemic tolerance of the immature kidney. Pediatr Nephrol. 18:85–91. [DOI] [PubMed] [Google Scholar]
- Xu L, Giffard RG. Hsp70 protects murine astrocytes from glucose deprivation injury. Neurosci Lett. 1997;224:9–12. doi: 10.1016/s0304-3940(97)13444-9.0304-3940(1997)224[0009:HPMAFG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yan LJ, Christians ES, Liu L, Xiao X, Sohal RS, Benjamin IJ. Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J. 2002;21:5164–5172. doi: 10.1093/emboj/cdf528.1460-2075(2002)021[5164:MHSTFD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007.0077-8923(2005)1053[0074:AAAMOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]