Abstract
Although granule cells continue to be added to the dentate gyrus of adult rats and tree shrews, this phenomenon has not been demonstrated in the dentate gyrus of adult primates. To determine whether neurons are produced in the dentate gyrus of adult primates, adult marmoset monkeys (Callithrix jacchus) were injected with BrdU and perfused 2 hr or 3 weeks later. BrdU is a thymidine analog that is incorporated into proliferating cells during S phase. A substantial number of cells in the dentate gyrus of adult monkeys incorporated BrdU and ≈80% of these cells had morphological characteristics of granule neurons and expressed a neuronal marker by the 3-week time point. Previous studies suggest that the proliferation of granule cell precursors in the adult dentate gyrus can be inhibited by stress in rats and tree shrews. To test whether an aversive experience has a similar effect on cell proliferation in the primate brain, adult marmoset monkeys were exposed to a resident-intruder model of stress. After 1 hr in this condition, the intruder monkeys were injected with BrdU and perfused 2 hr later. The number of proliferating cells in the dentate gyrus of the intruder monkeys was compared with that of unstressed control monkeys. We found that a single exposure to this stressful experience resulted in a significant reduction in the number of these proliferating cells. Our results suggest that neurons are produced in the dentate gyrus of adult monkeys and that the rate of precursor cell proliferation can be affected by a stressful experience.
In most regions of the mammalian brain, neurogenesis occurs during a discrete period of early development. In contrast, the hippocampal formation of the rat and tree shrew produces new granule neurons throughout adulthood (1–3). These new neurons originate from granule cell precursors that reside in the dentate gyrus and generate daughter cells that ultimately receive synaptic input (4, 5), extend axons into the mossy fiber pathway (6), and express a number of markers of mature granule neurons (1, 2). These findings raise the possibility that neurons continue to be produced naturally in the brains of primates, including humans, a phenomenon that would have implications for brain plasticity and repair. However, no previous study has demonstrated neurogenesis in the intact adult primate brain.
Our previous studies have shown that hormones of the adrenal gland, that are secreted in response to stress, suppress the proliferation of hippocampal granule cell precursors in the rat during development and in adulthood (7). Moreover, we have recently demonstrated that in tree shrews, animals considered to be phylogenetically between insectivores and primates, acute and chronic stressful experiences suppress the proliferation of precursor cells in the dentate gyrus (3, 8). Therefore, in addition to examining neurogenesis in the dentate gyrus of the adult marmoset monkey, we exposed the animals to a stressful experience and determined its acute effect on the proliferation of granule cell precursors.
MATERIALS AND METHODS
Animals.
Adult male common marmoset monkeys Callithrix jacchus (3 years of age) from the breeding colony at the German Primate Center (Göttingen, Germany) were used in all experiments. Marmoset monkeys are diurnal New World primates that reach sexual maturity at 14 months and have a lifespan of up to 12 years in captivity (9). All animal experimentation was approved by the Government of Lower Saxony, Germany, and was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animals were housed in peer groups on a 13-hr light photoperiod with artificial illumination from 8:00 AM to 9:00 PM in air conditioned rooms. All treatments were performed during the day (lights on).
Animal Treatments.
To determine whether neuronal precursors are present naturally in the dentate gyrus of adult primates, adult male marmoset monkeys (n = 6) were injected with the thymidine analog BrdU (Sigma; 75 mg/kg in saline with 0.007 N NaOH, this dose was used in all experiments), a marker of proliferating cells and their progeny (10). Two hours or 3 weeks after injection, the animals were transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB), the heads postfixed overnight, and the brains removed from the skulls on the following day. Coronal brain sections 40 μm thick were cut on an oscillating tissue slicer into a bath of PB and then processed for combined immunohistochemistry for BrdU and neuron-specific enolase (NSE). Although reactive astrocytes have been shown to express NSE in the injured rat brain (11), in the intact primate hippocampal formation human anti-NSE stains only cells with neuronal morphology. The two hour survival time was selected because it is sufficient for the uptake of BrdU but not for completion of mitosis or migration (1, 10). The 3-week survival time was selected because studies performed in the rat and tree shrew have shown that most cells in the dentate gyrus express the neuronal marker NSE by this time after DNA synthesis (1, 3).
To determine whether stressful experiences modulate the proliferation of granule cell precursors in the dentate gyrus, adult male marmoset monkeys were exposed to a resident-intruder model of stress as described below. This model has been used extensively for stress studies in rodents and monkeys and has been shown to increase physiological indicators of stress (12–14). In marmoset monkeys, transfer to an unfamiliar cage results in a clear activation of the sympathetic nervous system indicated by elevated mean arterial blood pressure and dramatically increased heart rate (C. R. Schnell, unpublished observation). Two hours following BrdU injection, the animals were deeply anesthetized and transcardially perfused. The heads were postfixed overnight and the brains removed from the skulls on the next day. Coronal brain sections 40-μm thick were cut on an oscillating tissue slicer into a bath of PB and processed for BrdU immunohistochemistry. The numbers of BrdU-labeled cells in the dentate gyrus of these animals were then compared with that observed in marmoset monkeys that were not exposed to a stressful experience but received a single BrdU injection followed by a 2-hr survival time.
Resident-Intruder Paradigm.
The experimental induction of resident-intruder stress was carried out as follows. Single male marmoset monkeys that had been housed individually were transferred into a smaller cage and placed within the home cage of an unfamiliar adult male conspecific. This resulted in an aggressive encounter wherein the intruder monkey assumed a subordinate position (14). Under these conditions, the intruder monkey remained motionless in the cage and avoided situations that could evoke attacks from the resident animal. The intruder monkey was then removed after 1 hr of exposure to the resident monkey, injected with BrdU, and perfused after a 2 hr survival time. These animals were compared with control marmoset monkeys that were treated similarly but not exposed to a resident monkey.
BrdU Labeling.
For BrdU immunohistochemistry, the sections were permeabilized with 0.5% (v/v) Triton X-100 in PBS for 1 hr, incubated with Pronase E (3 μg/ml) in PBS at 37°C for 20 min, denatured in 2 N HCl for 20 min, rinsed twice in PBS, and incubated overnight with anti-BrdU mAb (Novocastra, 1:100 in PB). The sections were rinsed in PBS, incubated for 1 hr in biotinylated mouse secondary antisera (Vector Laboratories, 1:50 in PBS), rinsed in PBS, incubated for 1 hr in avidin-biotin-horseradish peroxidase (Vector Laboratories, 1:50 in PBS) rinsed in PBS, and reacted with diaminobenzidine and hydrogen peroxide in PBS. After rinsing in PBS, the sections were mounted onto gelatinized glass slides and stained for Nissl using cresyl violet.
Combined BrdU and NSE Labeling.
For combined BrdU and NSE immunohistochemistry, the sections were stained for BrdU as described above using a nickel-cobalt-enhanced diaminobenzidine (to produce a black reaction product) and then incubated in anti-human polyclonal antisera to NSE (Polysciences, 1:2000 in PB). In the intact hippocampal formation of adult monkeys, NSE stains only cells with the morphological characteristics of neurons. After rinsing in PBS, the sections were incubated in biotinylated rabbit secondary antisera (Vector Laboratories, 1:50 in PBS), rinsed in PBS, incubated for 1 hr in avidin-biotin-horseradish peroxidase (Vector Laboratories, 1:50 in PBS), rinsed in PBS, and reacted in VIP diaminobenzidine (to produce a pink reaction product, Vector Laboratories). The sections were then mounted onto gelatinized slides, dehydrated, cleared, and coverslipped under Permount. Controls were processed as described above with omission of the primary antisera.
Data Analysis.
The slides were coded prior to quantitative analysis and the code was not broken until the analysis was complete. The number of BrdU-labeled cells was determined stereologically on randomly selected adjacent sections throughout the dentate gyrus. For each section, the number of BrdU-labeled cells in the dentate gyrus was determined avoiding counts of cells in the outermost plane of focus. The volume of the analyzed area was determined using Cavalieri’s principle (15) on video-projected images with cross-sectional area measurements performed using imagepro software (Media Cybernetics, Silver Spring, MD). The data were expressed as the number of BrdU-labeled cells per mm3). The overall volume of the dentate gyrus did not change with acute stress. Means were determined for each animal and the data were subjected to two-tailed Student’s t tests.
RESULTS
We found that a substantial number of precursor cells divide and differentiate into mature granule neurons in the dentate gyrus of adult marmoset monkeys. At the 2 hr post-BrdU injection time, many labeled cells were located throughout the rostrocaudal extent of the dentate gyrus (Figs. 1 and 2). The majority (>80%) of these cells had the morphological characteristics of granule cell precursors, i.e., round or oval cell bodies (Fig. 1), and were located in the subgranular zone, the region bordering the granule cell layer and hilus (Fig. 2). The remaining BrdU-labeled cells had the morphological characteristics of glial precursors, i.e., small, irregular or triangular shaped cell bodies. After a 3 week post-BrdU injection survival, the majority BrdU-labeled cells (>80%) in the dentate gyrus of adult marmoset monkeys were located in the granule cell layer and had the morphological characteristics of granule neurons, i.e., these cells were round or oval, and slightly larger than granule cell precursors (Fig. 1). At the 3 week time point, BrdU-labeled cells were evenly distributed throughout the rostrocaudal extent of the dentate gyrus (Fig. 2). Combined BrdU and NSE immunohistochemistry revealed that most (>80%) BrdU-labeled cells expressed the neuronal marker 3 weeks after DNA synthesis (Fig. 2).
Figure 1.
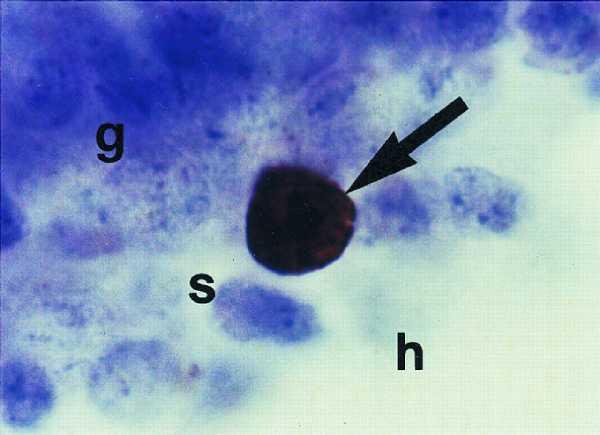
Photomicrograph of BrdU-labeled granule cell precursor in the dentate gyrus of an adult marmoset monkey. This cell is located on the border of the granule cell layer (g) and hilus (h), in the subgranular zone (s) and has the morphological characteristics of neighboring granule neurons (round and medium-sized cell body).
Figure 2.
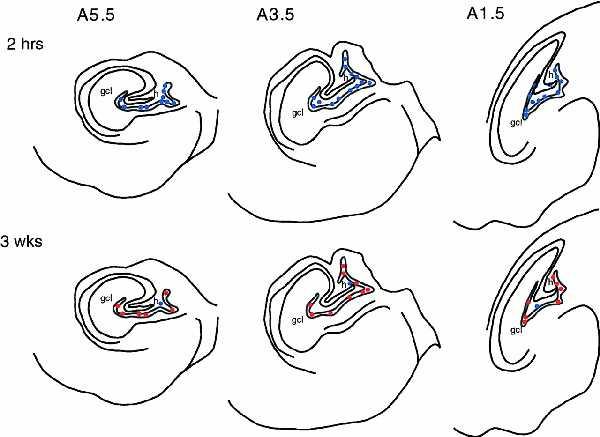
Templates of distribution of BrdU-labeled cells in the dentate gyrus of an adult marmoset monkey 2 hr and 3 weeks after BrdU injection. At 2 hr after BrdU injection, BrdU-labeled cells did not express the neuronal marker NSE. By 3 weeks following BrdU injection, the majority of BrdU-labeled cells express NSE. Blue dots represent BrdU+ NSE− cells. Red dots represent BrdU NSE+ cells.
Having found neurogenesis and differentiation of granule neurons in the adult marmoset monkey dentate gyrus, we investigated the effect of an acute psychosocial stress using a resident-intruder paradigm. We found that acute stress suppresses the proliferation of precursor cells in the dentate gyrus of adult marmoset monkeys. A single exposure to an unfamiliar adult male conspecific in its resident cage resulted in a significant decrease in the number of BrdU-labeled cells (Fig. 3). Furthermore, there were no differences in the distribution of BrdU-labeled cells throughout the rostrocaudal extent of the dentate gyrus following stress, indicating that the suppression of BrdU incorporation does not affect a subpopulation of cells in this brain region.
Figure 3.
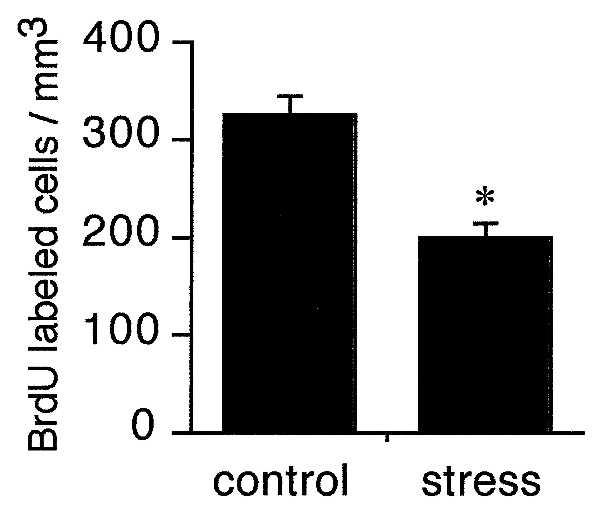
A single exposure to a resident-intruder model of stress results in a significant decrease in the number of BrdU-labeled cells in the dentate gyrus of the intruder marmoset monkey. Adult monkeys were placed individually in the home cage of a conspecific for 1 hr. After this time, the monkeys were removed and injected with BrdU, a marker of proliferating cells and their progeny. Two hours later, the monkeys were perfused and the brains processed for BrdU labeling. Bars = mean + SEM (n = 4, stress; n = 2 control). ∗, significant difference from control, P < 0.05 (unpaired Student’s t tests).
DISCUSSION
The results of this study indicate that a substantial number of proliferating cells exist in the dentate gyrus of adult monkeys and that at least some of these cells differentiate into mature granule neurons. Furthermore, our results indicate that the proliferation of precursor cells in the dentate gyrus can be affected by an acute, socially stressful experience. In the resident-intruder paradigm, the intruder animal shows physiological and behavioral indices of subordination (12–14). In another model of social stress, we have shown that subordinate tree shrews have diminished hippocampal cell proliferation compared with controls (3). In such paradigms, the dominant animal shows some signs of stress, although they are less severe than observed in the subordinate animals, e.g., slight elevations in glucocorticoid levels and decreases in body weight (16). Because it is likely that both resident and intruder monkeys are undergoing an aversive experience in the present paradigm, we chose to compare the group that showed the most dramatic signs of stress (intruder monkeys) to control monkeys. Comparing these two groups, we observed significantly lower numbers of proliferating cells in the dentate gyrus of resident monkeys.
It is likely that our results reflect a specific effect of stress, as opposed to a general effect of experience, on cell proliferation because a similar effect occurs in the rat dentate gyrus following exposure to an olfactory stressor, predator odor, where control animals have identical experiences without the odor (17). In addition, our studies using exposure to predator odor have shown no differences in the number of silver grains per 3H-thymidine-labeled cell in the dentate gyrus, despite lower numbers of labeled cells in the stressed group. These results imply that the decrease in number of labeled cells observed in the dentate gyrus following exposure to fox odor in rats, exposure to a dominant animal in tree shrews, or exposure to a resident animal in monkeys is the result of a decrease in the number of cells entering S phase, and not a change in the uptake of 3H-thymidine or BrdU. Although it is possible that stress resulted in a temporary lengthening of the cell cycle, as opposed to an inhibition of cell proliferation, our recent results showing that chronic subordination stress results in a persistent decrease in the number of proliferating cells in the dentate gyrus and an accompanying decrease in the volume of the granule cell layer in tree shrews (8), suggest that a stress-induced decrease in the number of BrdU-labeled cells reflects a decrease in the number of new granule neurons.
Neurogenesis in the dentate gyrus has been most extensively studied in the adult and developing rat brain. We have shown that the proliferation of granule cell precursors is suppressed by adrenal steroids and NMDA receptor-mediated excitatory input arising from the entorhinal cortex (18). Stress is known to elevate the levels of circulating adrenal steroids and stimulate the release of glutamate in the hippocampal formation (19–22). Thus, stress-induced suppression of cell proliferation is likely to involve both hormonal and neural factors.
Our observations that stress inhibits the proliferation of granule cell precursors in three different mammalian species— rat, tree shrew, and marmoset monkey—suggest that this phenomenon is a common characteristic of all animals that produce granule neurons in adulthood. Taken together with a report showing that precursor cells isolated from the adult human brain can produce neurons in vitro (23), our findings suggest that the human brain may produce neurons in adulthood as well. Thus, the stress-induced inhibition of cell proliferation in the marmoset monkey dentate gyrus raises questions about volume changes in the human dentate gyrus resulting from stress-related disorders. Indeed, recent studies indicate that elevated cortisol in Cushing disease (24), as well as recurrent depressive illness (25) and posttraumatic stress disorder are all associated with decreased volume of the hippocampus, as determined by MRI (26–28). The nature of the cellular changes in the hippocampus are presently unknown, that is, whether they represent reversible atrophy of neuronal processes, changes in the glial cell population or permanent loss of neurons (29). However, reduction in dentate gyrus volume as a result of stress and elevated adrenal steroids might make a contribution to the reduced hippocampal volume detected by MRI in these studies. Taken together with the present findings, it is possible that the reduced hippocampal volume can be partially attributed to chronically suppressed proliferation of neuronal precursors.
In conclusion, we have demonstrated neurogenesis in the dentate gyrus of an adult primate, the marmoset monkey, and shown that acute psychosocial stress inhibits the proliferation of granule cell precursors. Collectively with other data on the response of the hippocampal formation of rats, tree shrews, primates, and humans to stressful situations, these studies suggest that the production of granule neurons during adulthood and its inhibition by stressful experience may be common to many species, including humans.
Acknowledgments
We thank Fernando Nottebohm for helpful comments on the manuscript. This work was supported by Grant MH52423 (E.G.).
ABBREVIATIONS
- NSE
neuron-specific enolase
- PB
phosphate buffer
References
- 1.Cameron H A, Woolley C S, McEwen B S, Gould E. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn H G, Dickinson-Anson H, Gage F H. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould E, McEwen B S, Tanapat P, Galea L A M, Fuchs E. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan M S, Bell D H. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan M S, Hinds J W. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 6.Stanfield B B, Trice J E. Exp Brain Res. 1988;72:399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- 7.Gould E, Cameron H A. Dev Neurosci. 1996;18:22–35. doi: 10.1159/000111392. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs E, Flugge G, McEwen B S, Tanapat P, Gould E. Soc Neurosci Abstr. 1997;23:317. [Google Scholar]
- 9.Napier J R, Napier P H. A Handbook of Living Primates. New York: Academic; 1967. pp. 79–83. [Google Scholar]
- 10.Nowakowski R S, Lewin S B, Miller M W. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 11.Lin R C, Matesic D F. Neuroscience. 1994;60:11–16. doi: 10.1016/0306-4522(94)90199-6. [DOI] [PubMed] [Google Scholar]
- 12.Peterson J T, Pohorecky L A, Hamm M W. Pharmacol Biochem Behav. 1989;34:247–253. doi: 10.1016/0091-3057(89)90307-9. [DOI] [PubMed] [Google Scholar]
- 13.Sgoifo A, de Boer S F, Haller J, Koolhaas J M. Physiol Behav. 1996;60:1403–1407. doi: 10.1016/s0031-9384(96)00229-6. [DOI] [PubMed] [Google Scholar]
- 14.Miczek K A, Yoshimura H. Psychopharmacology. 1982;76:163–171. doi: 10.1007/BF00435272. [DOI] [PubMed] [Google Scholar]
- 15.Gundersen H J G, Bendtsen T F, Korbo L, Marcussen N, Moller A, Kielsen K, Pakkenberg B, Sorensen F B, Vesterby A, West M J. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 16.Magarinos A M, McEwen B S, Flügge G, Fuchs E. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galea L A M, Tanapat P, Gould E. Soc Neurosci Abstr. 1996;22:1196. [Google Scholar]
- 18.Cameron H A, McEwen B S, Gould E. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moghaddam B. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- 20.Lowy M T, Gault L, Yamamoto B K. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- 21.Moghaddam B, Boliano M L, Stein-Behrens B, Sapolsky R. Brain Res. 1994;655:251–254. doi: 10.1016/0006-8993(94)91622-5. [DOI] [PubMed] [Google Scholar]
- 22.De Bruin L A, Schasfoort M C, Stefens A B, Korf J. Am J Physiol. 1994;259:R773–R779. doi: 10.1152/ajpregu.1990.259.4.R773. [DOI] [PubMed] [Google Scholar]
- 23.Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser R A, Goldman S A. Cereb Cortex. 1994;4:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 24.Starkman M N, Gebarski S S, Berent S, Schteingart D E. Biol Psychiatr. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 25.Sheline Y I, Wang P W, Gado M H, Csernansky J C, Vannier M W. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bremner D J, Randall P, Scott T M, Bronen R A, Seibyl J P, Southwick S M, Delaney R C, McCarthy G, Charney D S, Innis R B. Am J Psychiat. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurvits T V, Shenton M E, Hokama H, Ohta H, Lasko N B, Gilbertson M W, Orr S P, Kikinis R, Jolesz F A, McCarley R W, et al. Biol Psychiatr. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bremner J D, Randall P, Vermetten E, Staib L, Bronen R A, Mazure C, Capelli S, McCarthy G, Innis R B, Charney D S. Biol Psychiatr. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEwen B S. Mol Psychiatr. 1997;2:255–262. doi: 10.1038/sj.mp.4000254. [DOI] [PubMed] [Google Scholar]


