Abstract
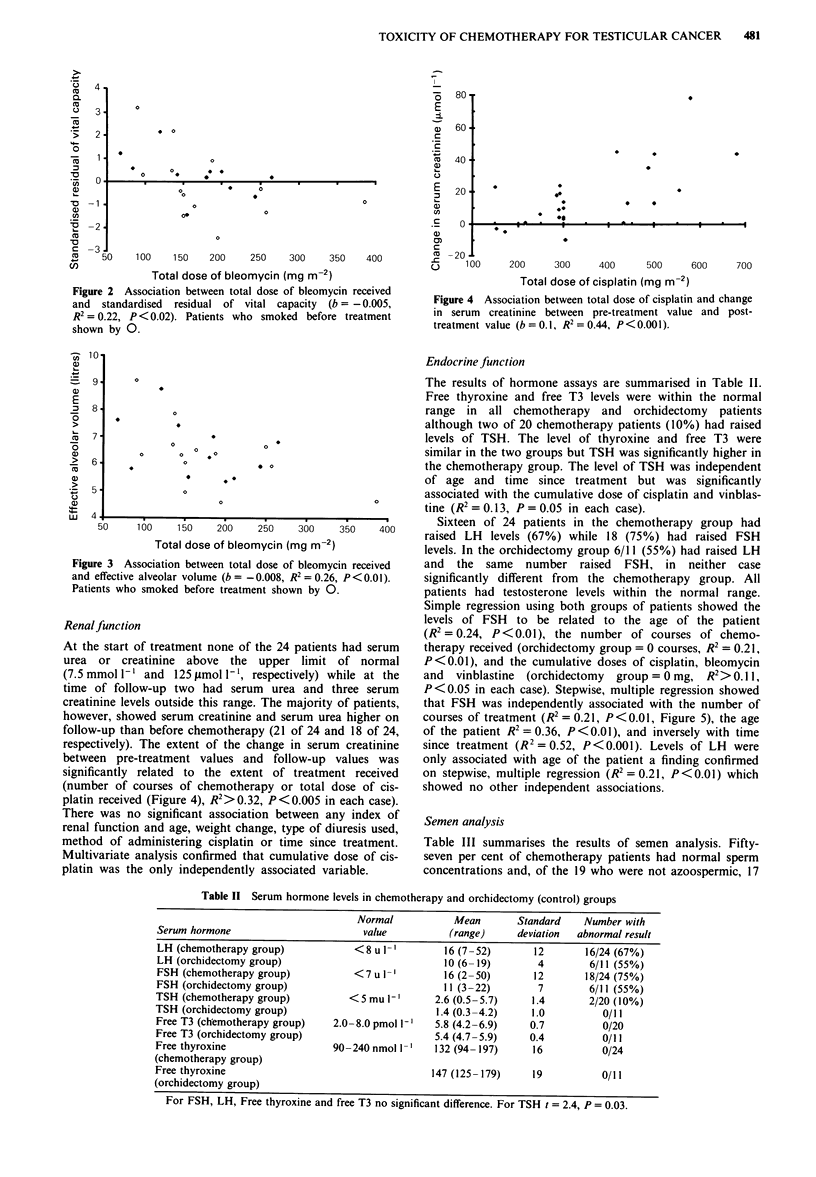
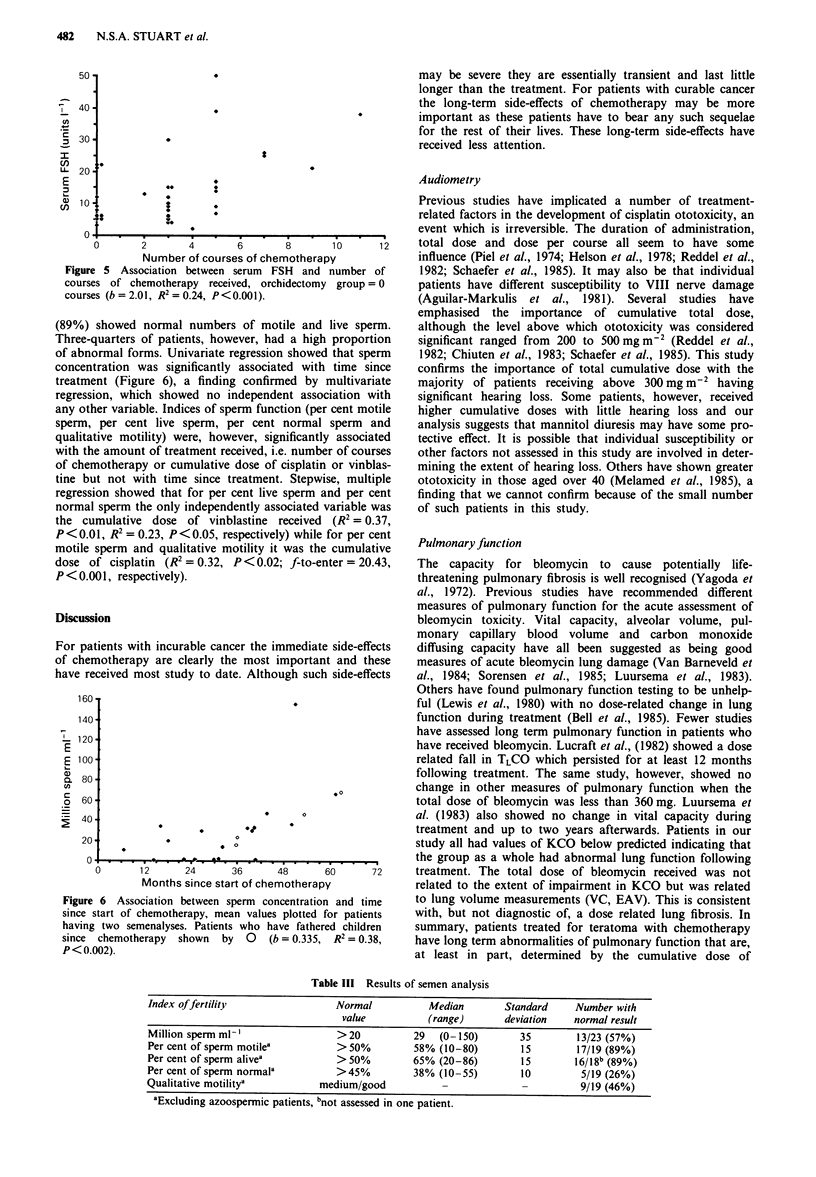
Twenty-seven patients cured of advanced testicular cancer by cisplatin-based chemotherapy have been assessed, a median of 30 months after start of treatment, for the long-term effects of such treatment on renal, endocrine, audiometric, reproductive and respiratory function. To control for the effects of orchidectomy on endocrine function a similar group of 11 patients cured by orchidectomy alone was also assessed. The extents of impairment in hearing and renal function were related to the total dose of cisplatin received, while the majority of patients had respiratory impairment which was, in part, related to the total dose of bleomycin. TSH was significantly higher in the chemotherapy group although serum free thyroxine and free T3 were normal in all. FSH was raised in 67% of the chemotherapy group although serum free thyroxine and free T3 were while LH was raised in 75% and 45% respectively. Serum testosterone was normal in all. The levels of FSH and LH were both independently correlated with age of the patient while FSH was higher in patients having more chemotherapy and had a tendency to fall towards normal with time since treatment. Over half the patients had normal sperm concentrations although 74% had a raised proportion of abnormal sperm. Indices of sperm function were worse in patients having more chemotherapy but sperm number increased towards normal with time since treatment, particularly after the second year. The long-term side-effects of chemotherapy for testicular cancer are thus generally mild but are largely irreversible and their severity is related to the total amount of chemotherapy received. As their longer term significance is not clear we would recommend that, in the treatment of testicular cancer, doses of chemotherapy are reduced to the minimum required for cure. Assessment of long-term side-effects of chemotherapy for testicular cancer should be a mandatory part of any study of such treatment and should be considered in any comparison of different therapies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Markulis N. V., Beckley S., Priore R., Mettlin C. Auditory toxicity effects of long-term cis-dichlorodiammineplatinum II therapy in genitourinary cancer patients. J Surg Oncol. 1981;16(2):111–123. doi: 10.1002/jso.2930160203. [DOI] [PubMed] [Google Scholar]
- Bell M. R., Meredith D. J., Gill P. G. Role of carbon monoxide diffusing capacity in the early detection of major bleomycin-induced pulmonary toxicity. Aust N Z J Med. 1985 Apr;15(2):235–240. doi: 10.1111/j.1445-5994.1985.tb04015.x. [DOI] [PubMed] [Google Scholar]
- Chiuten D., Vogl S., Kaplan B., Camacho F. Is there cumulative or delayed toxicity from cis-platinum? Cancer. 1983 Jul 15;52(2):211–214. doi: 10.1002/1097-0142(19830715)52:2<211::aid-cncr2820520205>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Daugaard G., Rossing N., Rørth M. Effects of cisplatin on different measures of glomerular function in the human kidney with special emphasis on high-dose. Cancer Chemother Pharmacol. 1988;21(2):163–167. doi: 10.1007/BF00257365. [DOI] [PubMed] [Google Scholar]
- Davies J. M. Testicular cancer in England and Wales: some epidemiological aspects. Lancet. 1981 Apr 25;1(8226):928–932. doi: 10.1016/s0140-6736(81)91625-1. [DOI] [PubMed] [Google Scholar]
- Einhorn L. H., Williams S. D. Chemotherapy of disseminated testicular cancer. A random prospective study. Cancer. 1980 Sep 15;46(6):1339–1344. doi: 10.1002/1097-0142(19800915)46:6<1339::aid-cncr2820460607>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Fosså S. D., Klepp O., Aakvaag A. Serum hormone levels in patients with malignant testicular germ cell tumours without clinical and/or radiological signs of tumour. Br J Urol. 1980 Apr;52(2):151–157. doi: 10.1111/j.1464-410x.1980.tb02946.x. [DOI] [PubMed] [Google Scholar]
- Goren M. P., Wright R. K., Horowitz M. E. Cumulative renal tubular damage associated with cisplatin nephrotoxicity. Cancer Chemother Pharmacol. 1986;18(1):69–73. doi: 10.1007/BF00253068. [DOI] [PubMed] [Google Scholar]
- Helson L., Okonkwo E., Anton L., Cvitkovic E. cis-Platinum ototoxicity. Clin Toxicol. 1978;13(4):469–478. doi: 10.3109/15563657808988252. [DOI] [PubMed] [Google Scholar]
- Johnson D. H., Hainsworth J. D., Linde R. B., Greco F. A. Testicular function following combination chemotherapy with cis-platin, vinblastine, and bleomycin. Med Pediatr Oncol. 1984;12(4):233–238. doi: 10.1002/mpo.2950120403. [DOI] [PubMed] [Google Scholar]
- Lange P. H., Narayan P., Vogelzang N. J., Shafer R. B., Kennedy B. J., Fraley E. E. Return of fertility after treatment for nonseminomatous testicular cancer: changing concepts. J Urol. 1983 Jun;129(6):1131–1135. doi: 10.1016/s0022-5347(17)52607-5. [DOI] [PubMed] [Google Scholar]
- Leitner S. P., Bosl G. J., Bajorunas D. Gonadal dysfunction in patients treated for metastatic germ-cell tumors. J Clin Oncol. 1986 Oct;4(10):1500–1505. doi: 10.1200/JCO.1986.4.10.1500. [DOI] [PubMed] [Google Scholar]
- Lewis B. M., Izbicki R. Routine pulmonary function tests during bleomycin therapy. Tests may be ineffective and potentially misleading. JAMA. 1980 Jan 25;243(4):347–351. [PubMed] [Google Scholar]
- Lucraft H. H., Wilkinson P. M., Stretton T. B., Read G. Role of pulmonary function tests in the prevention of bleomycin pulmonary toxicity during chemotherapy for metastatic testicular teratoma. Eur J Cancer Clin Oncol. 1982 Feb;18(2):133–139. doi: 10.1016/0277-5379(82)90056-6. [DOI] [PubMed] [Google Scholar]
- Luursema P. B., Star-Kroesen M. A., van der Mark T. W., Sleyfer D. T., Koops H. S., Peset R. Bleomycin-induced changes in the carbon monoxide transfer factor of the lungs and its components. Am Rev Respir Dis. 1983 Nov;128(5):880–883. doi: 10.1164/arrd.1983.128.5.880. [DOI] [PubMed] [Google Scholar]
- Melamed L. B., Selim M. A., Schuchman D. Cisplatin ototoxicity in gynecologic cancer patients. A preliminary report. Cancer. 1985 Jan 1;55(1):41–43. doi: 10.1002/1097-0142(19850101)55:1<41::aid-cncr2820550106>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Miller M. R., Pincock A. C. Predicted values: how should we use them? Thorax. 1988 Apr;43(4):265–267. doi: 10.1136/thx.43.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlands E. S., Begent R. H., Kaye S. B., Rustin G. J., Bagshawe K. D. Chemotherapy of advanced malignant teratomas. Br J Cancer. 1980 Sep;42(3):378–391. doi: 10.1038/bjc.1980.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R. B. Creatinine clearance: a redundant clinical investigation. Ann Clin Biochem. 1986 May;23(Pt 3):243–250. doi: 10.1177/000456328602300304. [DOI] [PubMed] [Google Scholar]
- Piel I. J., Meyer D., Perlia C. P., Wolfe V. I. Effects of cis-diamminedichloroplatinum (NSC-119875) on hearing function in man. Cancer Chemother Rep. 1974 Nov-Dec;58(6):871–875. [PubMed] [Google Scholar]
- Reddel R. R., Kefford R. F., Grant J. M., Coates A. S., Fox R. M., Tattersall M. H. Ototoxicity in patients receiving cisplatin: importance of dose and method of drug administration. Cancer Treat Rep. 1982 Jan;66(1):19–23. [PubMed] [Google Scholar]
- Reece P. A., Stafford I., Russell J., Khan M., Gill P. G. Creatinine clearance as a predictor of ultrafilterable platinum disposition in cancer patients treated with cisplatin: relationship between peak ultrafilterable platinum plasma levels and nephrotoxicity. J Clin Oncol. 1987 Feb;5(2):304–309. doi: 10.1200/JCO.1987.5.2.304. [DOI] [PubMed] [Google Scholar]
- Schaefer S. D., Post J. D., Close L. G., Wright C. G. Ototoxicity of low- and moderate-dose cisplatin. Cancer. 1985 Oct 15;56(8):1934–1939. doi: 10.1002/1097-0142(19851015)56:8<1934::aid-cncr2820560807>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Stewart D. J., Mikhael N. Z., Nanji A. A., Nair R. C., Kacew S., Howard K., Hirte W., Maroun J. A. Renal and hepatic concentrations of platinum: relationship to cisplatin time, dose, and nephrotoxicity. J Clin Oncol. 1985 Sep;3(9):1251–1256. doi: 10.1200/JCO.1985.3.9.1251. [DOI] [PubMed] [Google Scholar]
- Swainson C. P., Colls B. M., Fitzharris B. M. Cis-platinum and distal renal tubule toxicity. N Z Med J. 1985 May 22;98(779):375–378. [PubMed] [Google Scholar]
- Sørensen P. G., Rossing N., Rørth M. Carbon monoxide diffusing capacity: a reliable indicator of bleomycin-induced pulmonary toxicity. Eur J Respir Dis. 1985 May;66(5):333–340. [PubMed] [Google Scholar]
- Van Barneveld P. W., van der Mark T. W., Sleijfer D. T., Mulder N. H., Koops H. S., Sluiter H. J., Peset R. Predictive factors for bleomycin-induced pneumonitis. Am Rev Respir Dis. 1984 Dec;130(6):1078–1081. doi: 10.1164/arrd.1984.130.6.1078. [DOI] [PubMed] [Google Scholar]
- Yagoda A., Mukherji B., Young C., Etcubanas E., Lamonte C., Smith J. R., Tan C. T., Krakoff I. H. Bleomycin, an antitumor antibiotic. Clinical experience in 274 patients. Ann Intern Med. 1972 Dec;77(6):861–870. doi: 10.7326/0003-4819-77-6-861. [DOI] [PubMed] [Google Scholar]


