Abstract
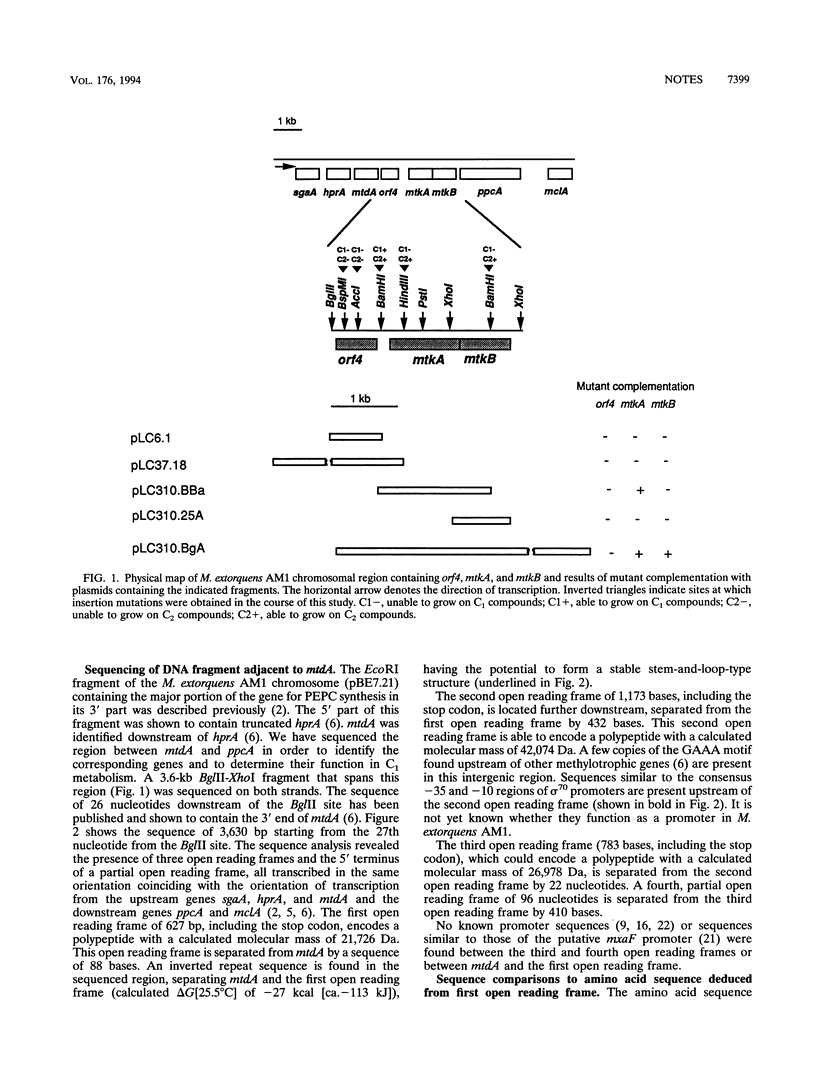
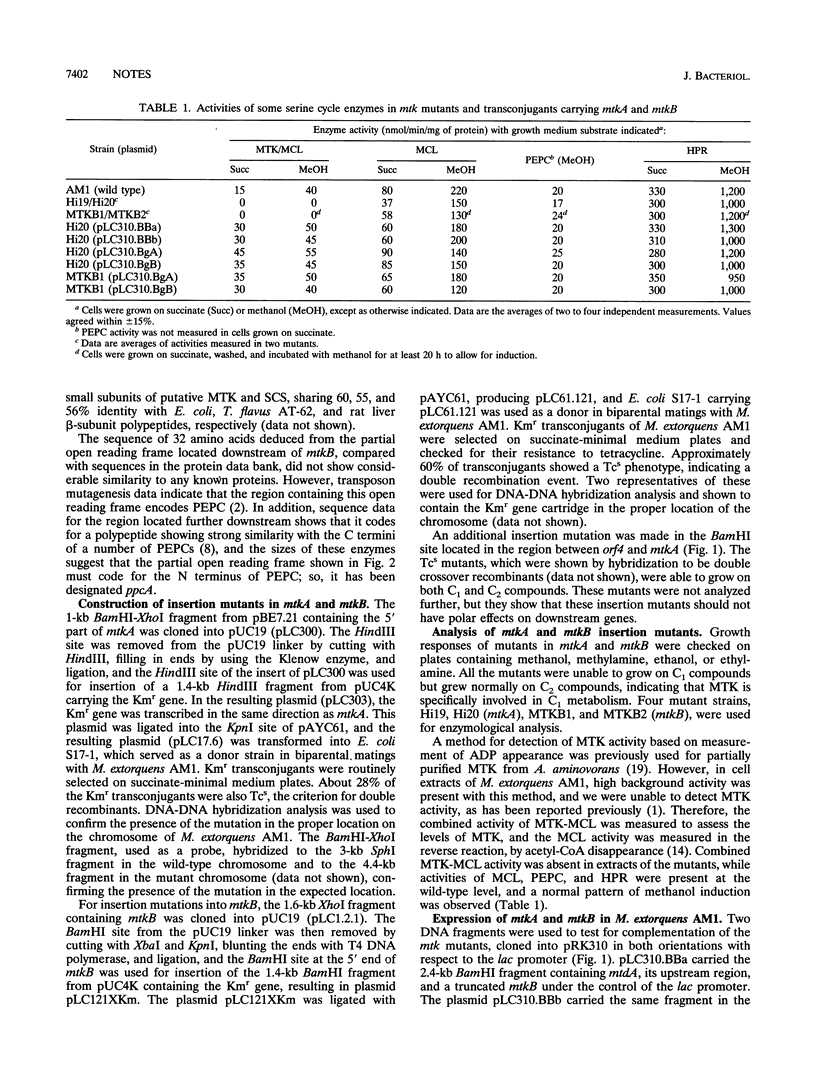
In a recent paper we reported the sequence of the beginning of a serine cycle gene cluster on the Methylobacterium extorquens AM1 chromosome, containing the genes encoding serine glyoxylate aminotransferase (sgaA), hydroxypyruvate reductase (hprA), and 5,10-methylenetetrahydrofolate dehydrogenase (mtdA) (L. V. Chistoserdova and M. E. Lidstrom J. Bacteriol. 176:1957-1968, 1994). Here we present the sequence of the adjacent downstream region containing three full and one partial open reading frames. The first of the full open reading frames (orf4) remains unidentified, while the other two (mtkA and mtkB) code for the two subunits of malate thiokinase, and the fourth, a partial open reading frame (ppcA), apparently encodes phosphoenolpyruvate carboxylase. Mutants containing insertion mutations in orf4, mtdA, and mtdB all were unable to grow on C1 compounds, showing that these three newly identified genes are indispensable for the operation of the serine cycle. Mutants in orf4 were also unable to grow on C2 compounds, but growth was restored by glyoxylate, suggesting that orf4 might be required for the conversion of acetyl coenzyme A to glyoxylate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arps P. J., Fulton G. F., Minnich E. C., Lidstrom M. E. Genetics of serine pathway enzymes in Methylobacterium extorquens AM1: phosphoenolpyruvate carboxylase and malyl coenzyme A lyase. J Bacteriol. 1993 Jun;175(12):3776–3783. doi: 10.1128/jb.175.12.3776-3783.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck D., Spencer M. E., Guest J. R. Primary structure of the succinyl-CoA synthetase of Escherichia coli. Biochemistry. 1985 Oct 22;24(22):6245–6252. doi: 10.1021/bi00343a031. [DOI] [PubMed] [Google Scholar]
- Chistoserdov A. Y., Chistoserdova L. V., McIntire W. S., Lidstrom M. E. Genetic organization of the mau gene cluster in Methylobacterium extorquens AM1: complete nucleotide sequence and generation and characteristics of mau mutants. J Bacteriol. 1994 Jul;176(13):4052–4065. doi: 10.1128/jb.176.13.4052-4065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistoserdova L. V., Lidstrom M. E. Cloning, mutagenesis, and physiological effect of a hydroxypyruvate reductase gene from Methylobacterium extorquens AM1. J Bacteriol. 1992 Jan;174(1):71–77. doi: 10.1128/jb.174.1.71-77.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistoserdova L. V., Lidstrom M. E. Genetics of the serine cycle in Methylobacterium extorquens AM1: cloning, sequence, mutation, and physiological effect of glyA, the gene for serine hydroxymethyltransferase. J Bacteriol. 1994 Nov;176(21):6759–6762. doi: 10.1128/jb.176.21.6759-6762.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistoserdova L. V., Lidstrom M. E. Genetics of the serine cycle in Methylobacterium extorquens AM1: identification of sgaA and mtdA and sequences of sgaA, hprA, and mtdA. J Bacteriol. 1994 Apr;176(7):1957–1968. doi: 10.1128/jb.176.7.1957-1968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Dunstan P. M., Anthony C., Drabble W. T. Microbial metabolism of C 1 and C 2 compounds. The involvement of glycollate in the metabolism of ethanol and of acetate by Pseudomonas AM1. Biochem J. 1972 Jun;128(1):99–106. doi: 10.1042/bj1280099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan P. M., Anthony C., Drabble W. T. Microbial metabolism of C 1 and C 2 compounds. The role of glyoxylate, glycollate and acetate in the growth of Pseudomonas AM1 on ethanol and on C 1 compounds. Biochem J. 1972 Jun;128(1):107–115. doi: 10.1042/bj1280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton G. L., Nunn D. N., Lidstrom M. E. Molecular cloning of a malyl coenzyme A lyase gene from Pseudomonas sp. strain AM1, a facultative methylotroph. J Bacteriol. 1984 Nov;160(2):718–723. doi: 10.1128/jb.160.2.718-723.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- Henning W. D., Upton C., McFadden G., Majumdar R., Bridger W. A. Cloning and sequencing of the cytoplasmic precursor to the alpha subunit of rat liver mitochondrial succinyl-CoA synthetase. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1432–1436. doi: 10.1073/pnas.85.5.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh L. B. Malate adenosine triphosphate lyase. Separation of the reaction into a malate thiokinase and malyl coenzyme A lyase. J Biol Chem. 1973 Nov 10;248(21):7295–7303. [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Lidstrom M. E., Tsygankov Y. D. Molecular genetics of methylotrophic bacteria. Biotechnology. 1991;18:273–304. doi: 10.1016/b978-0-7506-9188-8.50019-x. [DOI] [PubMed] [Google Scholar]
- Lonetto M., Gribskov M., Gross C. A. The sigma 70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992 Jun;174(12):3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Nishiyama M., Horinouchi S., Beppu T. Characterization of an operon encoding succinyl-CoA synthetase and malate dehydrogenase from Thermus flavus AT-62 and its expression in Escherichia coli. Mol Gen Genet. 1991 Apr;226(1-2):1–9. doi: 10.1007/BF00273580. [DOI] [PubMed] [Google Scholar]
- PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. I. Isolation and characterization of Pseudomonas AM 1. Biochem J. 1961 Dec;81:465–469. doi: 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Tsuji K., Tsien H. C., Hanson R. S., DePalma S. R., Scholtz R., LaRoche S. 16S ribosomal RNA sequence analysis for determination of phylogenetic relationship among methylotrophs. J Gen Microbiol. 1990 Jan;136(1):1–10. doi: 10.1099/00221287-136-1-1. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Whitaker J. R., Granum P. E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980 Nov 15;109(1):156–159. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]



