Abstract
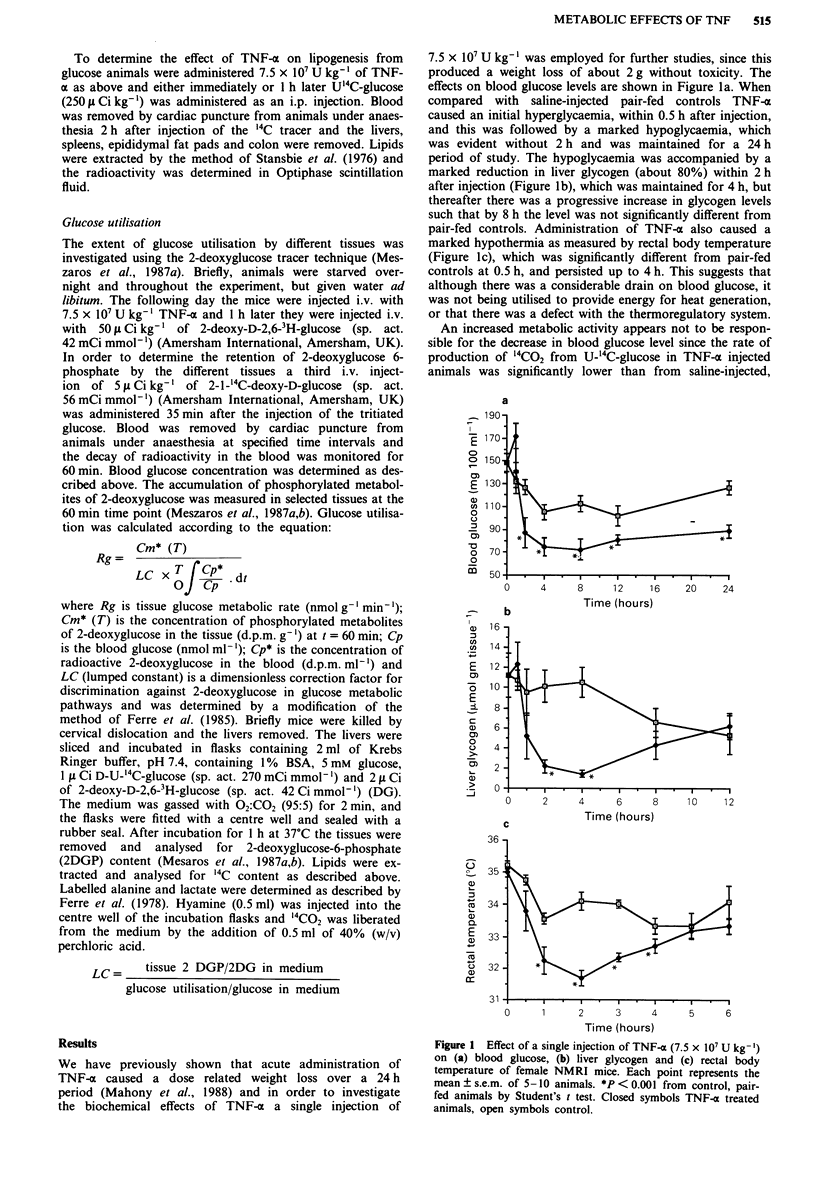
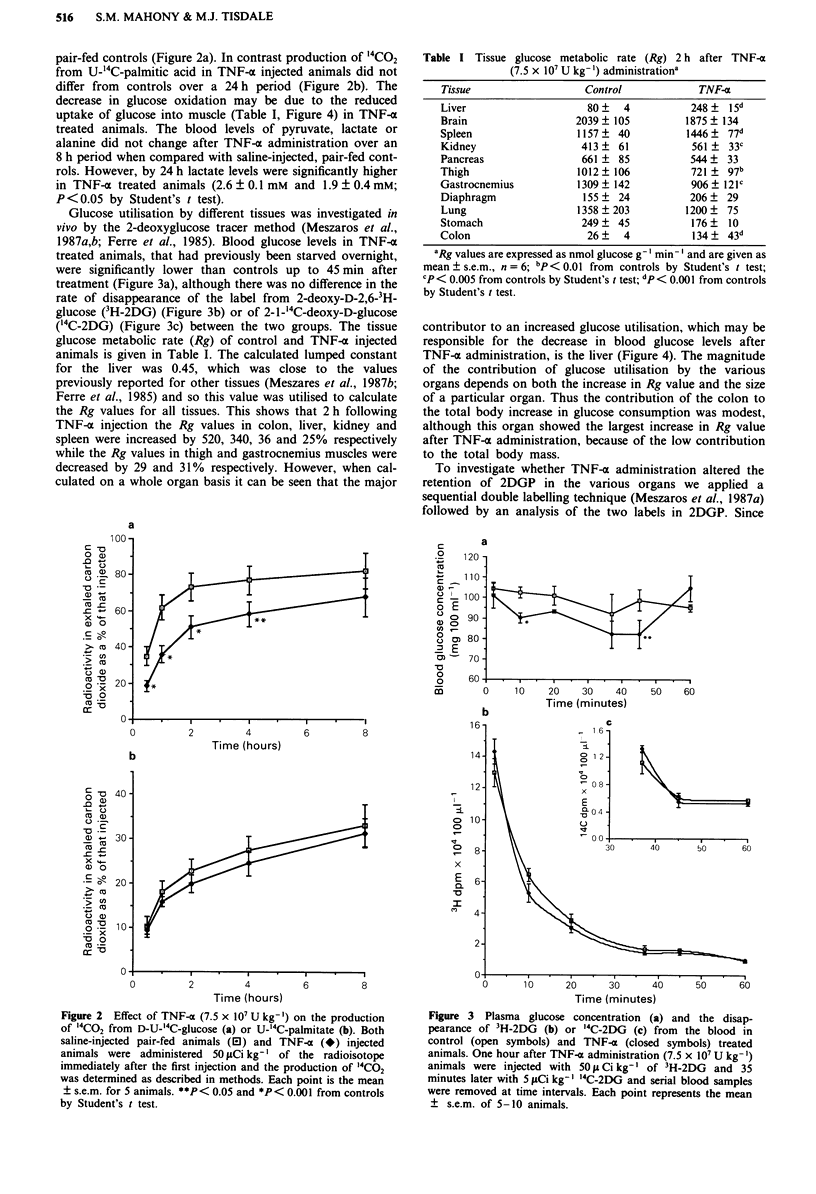
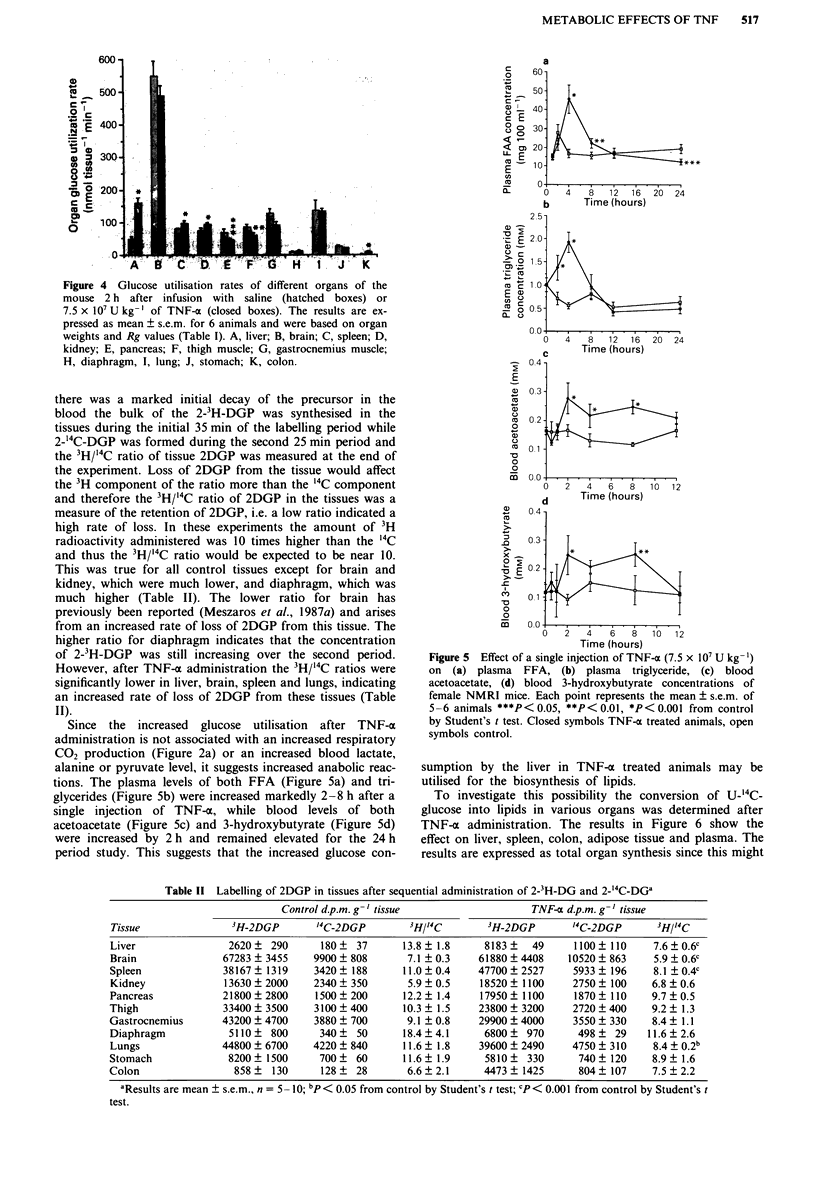
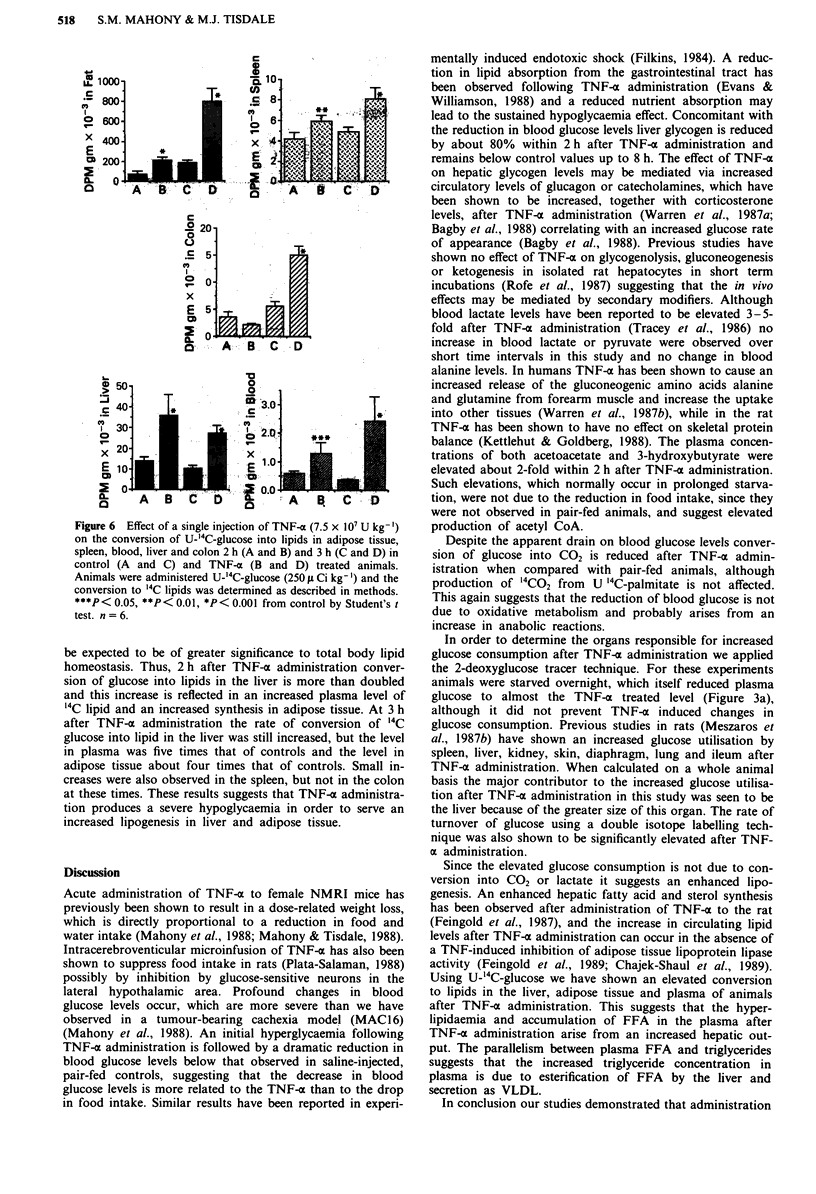
Following a single injection of 7.5 x 10(7) U kg-1 of human recombinant tumour necrosis factor-alpha (TNF-alpha) to female NMRI mice, marked hypoglycaemia was observed within a 2 h period, accompanied by a severe depletion of liver glycogen and a drop in rectal body temperature when compared with pair-fed controls. There was no alteration in plasma alanine, lactate or pyruvate values, but an elevation of acetoacetate and 3-hydroxybutyrate when compared with pair-fed controls. Production of 14CO2 from U-14C-glucose was reduced in TNF-alpha treated animals, while production of 14CO2 from U-14C-palmitate was not significantly different from controls, suggesting that the glucose was not being used to provide an increased metabolic rate. Glucose utilisation by different tissues was investigated by the 2-deoxyglucose tracer method. This showed that 2 h following TNF-alpha infusion glucose utilisation was increased in colon, liver, kidney and spleen by 500, 350, 36 and 25% respectively. However, when calculated on a whole-animal basis the major contributor to the increased glucose consumption was the liver. Plasma levels of both FFA and triglycerides were also elevated in TNF-alpha treated animals, suggesting that increased consumption of glucose by the liver may be utilised for lipogenesis. The rate of conversion of glucose into lipids in the liver was more than doubled 2 h after TNF-alpha administration with a concomitant rise in plasma and adipose tissue. These results suggest that administration of TNF-alpha produces a severe hypoglycaemia in order to serve an increased lipogenesis in liver and adipose tissue, which appears to be independent of the anorectic effect.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Cerami A., Ikeda Y., Le Trang N., Hotez P. J., Beutler B. Weight loss associated with an endotoxin-induced mediator from peritoneal macrophages: the role of cachectin (tumor necrosis factor). Immunol Lett. 1985;11(3-4):173–177. doi: 10.1016/0165-2478(85)90165-8. [DOI] [PubMed] [Google Scholar]
- Chajek-Shaul T., Friedman G., Stein O., Shiloni E., Etienne J., Stein Y. Mechanism of the hypertriglyceridemia induced by tumor necrosis factor administration to rats. Biochim Biophys Acta. 1989 Feb 20;1001(3):316–324. doi: 10.1016/0005-2760(89)90116-1. [DOI] [PubMed] [Google Scholar]
- Evans R. D., Williamson D. H. Tumour necrosis factor alpha (cachectin) mimics some of the effects of tumour growth on the disposal of a [14C]lipid load in virgin, lactating and litter-removed rats. Biochem J. 1988 Dec 15;256(3):1055–1058. doi: 10.1042/bj2561055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K. R., Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987 Jul;80(1):184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K. R., Soued M., Staprans I., Gavin L. A., Donahue M. E., Huang B. J., Moser A. H., Gulli R., Grunfeld C. Effect of tumor necrosis factor (TNF) on lipid metabolism in the diabetic rat. Evidence that inhibition of adipose tissue lipoprotein lipase activity is not required for TNF-induced hyperlipidemia. J Clin Invest. 1989 Apr;83(4):1116–1121. doi: 10.1172/JCI113991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré P., Leturque A., Burnol A. F., Penicaud L., Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J. 1985 May 15;228(1):103–110. doi: 10.1042/bj2280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré P., Pegorier J. P., Marliss E. B., Girard J. R. Influence of exogenous fat and gluconeogenic substrates on glucose homeostasis in the newborn rat. Am J Physiol. 1978 Feb;234(2):E129–E136. doi: 10.1152/ajpendo.1978.234.2.E129. [DOI] [PubMed] [Google Scholar]
- Filkins J. P. Reticuloendothelial system function and glucose-insulin dyshomeostasis in sepsis. Am J Emerg Med. 1984 Jan;2(1):70–73. doi: 10.1016/0735-6757(84)90111-6. [DOI] [PubMed] [Google Scholar]
- Kettelhut I. C., Goldberg A. L. Tumor necrosis factor can induce fever in rats without activating protein breakdown in muscle or lipolysis in adipose tissue. J Clin Invest. 1988 May;81(5):1384–1389. doi: 10.1172/JCI113467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony S. M., Beck S. A., Tisdale M. J. Comparison of weight loss induced by recombinant tumour necrosis factor with that produced by a cachexia-inducing tumour. Br J Cancer. 1988 Apr;57(4):385–389. doi: 10.1038/bjc.1988.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony S. M., Tisdale M. J. Induction of weight loss and metabolic alterations by human recombinant tumour necrosis factor. Br J Cancer. 1988 Sep;58(3):345–349. doi: 10.1038/bjc.1988.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mészáros K., Lang C. H., Bagby G. J., Spitzer J. J. Tumor necrosis factor increases in vivo glucose utilization of macrophage-rich tissues. Biochem Biophys Res Commun. 1987 Nov 30;149(1):1–6. doi: 10.1016/0006-291x(87)91596-8. [DOI] [PubMed] [Google Scholar]
- Oliff A., Defeo-Jones D., Boyer M., Martinez D., Kiefer D., Vuocolo G., Wolfe A., Socher S. H. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987 Aug 14;50(4):555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán C. R., Oomura Y., Kai Y. Tumor necrosis factor and interleukin-1 beta: suppression of food intake by direct action in the central nervous system. Brain Res. 1988 May 10;448(1):106–114. doi: 10.1016/0006-8993(88)91106-7. [DOI] [PubMed] [Google Scholar]
- Rofe A. M., Conyers R. A., Bais R., Gamble J. R., Vadas M. A. The effects of recombinant tumour necrosis factor (cachectin) on metabolism in isolated rat adipocyte, hepatocyte and muscle preparations. Biochem J. 1987 Nov 1;247(3):789–792. doi: 10.1042/bj2470789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. L., Spriggs D. R., Arthur K. A., Imamura K., Frei E., 3rd, Kufe D. W. Recombinant human tumor necrosis factor administered as a five-day continuous infusion in cancer patients: phase I toxicity and effects on lipid metabolism. J Clin Oncol. 1988 Feb;6(2):344–350. doi: 10.1200/JCO.1988.6.2.344. [DOI] [PubMed] [Google Scholar]
- Socher S. H., Martinez D., Craig J. B., Kuhn J. G., Oliff A. Tumor necrosis factor not detectable in patients with clinical cancer cachexia. J Natl Cancer Inst. 1988 Jun 15;80(8):595–598. doi: 10.1093/jnci/80.8.595. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Wei H., Manogue K. R., Fong Y., Hesse D. G., Nguyen H. T., Kuo G. C., Beutler B., Cotran R. S., Cerami A. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988 Mar 1;167(3):1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. S., Donner D. B., Starnes H. F., Jr, Brennan M. F. Modulation of endogenous hormone action by recombinant human tumor necrosis factor. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8619–8622. doi: 10.1073/pnas.84.23.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. S., Starnes H. F., Jr, Gabrilove J. L., Oettgen H. F., Brennan M. F. The acute metabolic effects of tumor necrosis factor administration in humans. Arch Surg. 1987 Dec;122(12):1396–1400. doi: 10.1001/archsurg.1987.01400240042007. [DOI] [PubMed] [Google Scholar]


