Abstract
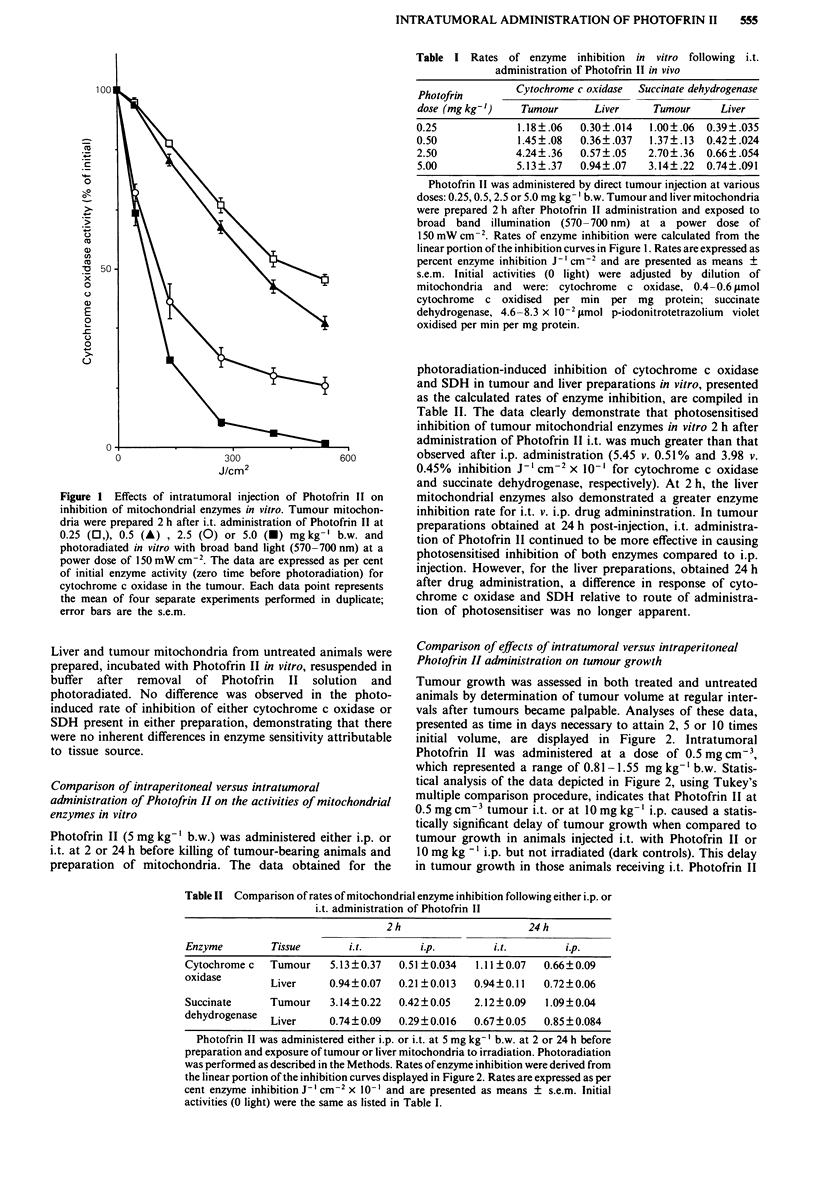
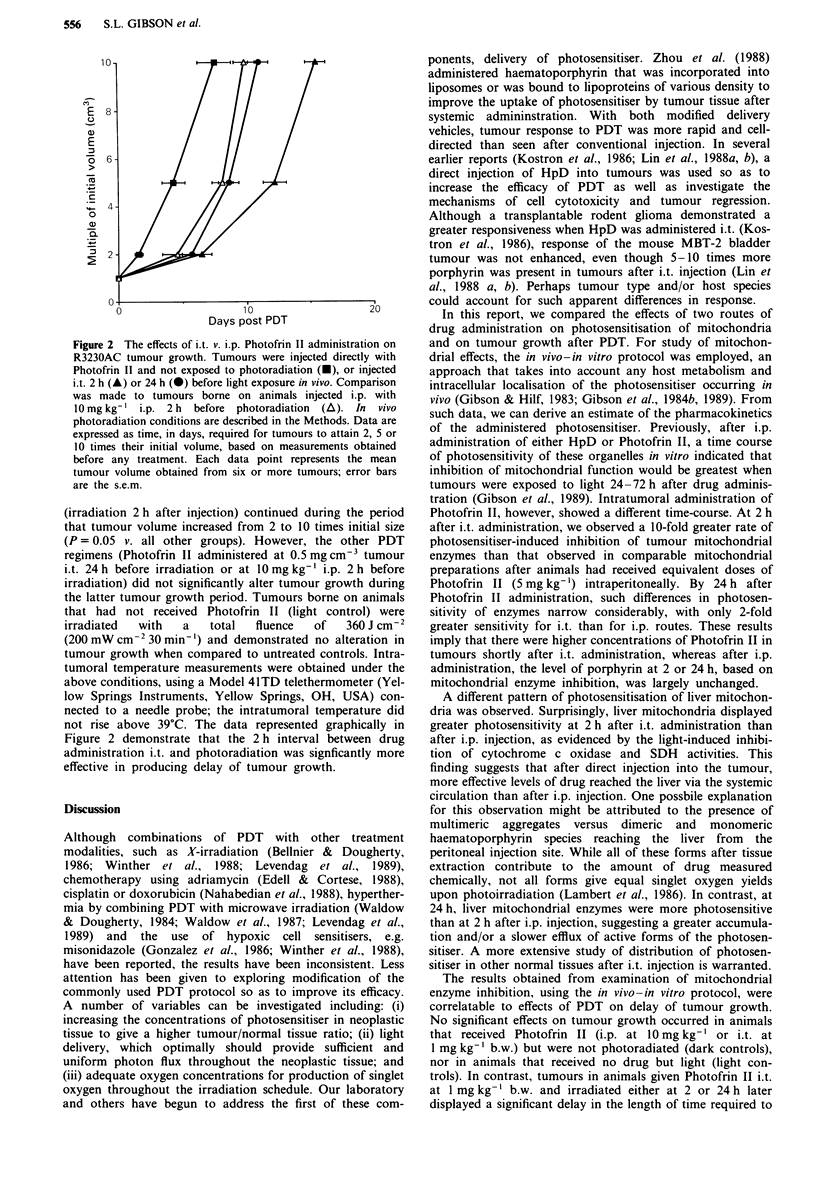
Photodynamic therapy consists of the systemic administration of a derivative of haematoporphyrin (Photofrin II) followed 24-72 h later by exposure of malignant lesions to photoradiation. We investigated the efficacy of this treatment after direct intratumoral injection of Photofrin II. This direct treatment regimen resulted in higher rates of inhibition of mitochondrial cytochrome c oxidase (5.13% J-1 cm-2 x 10(-1) and succinate dehydrogenase (3.14% J-1 cm-2 x 10(-1] in vitro at 2 h after intratumoral injection compared to rates of inhibition obtained after intraperitoneal drug administration: 0.51 and 0.42% J-1 cm-2 x 10(-1), respectively. A significant delay in tumour growth in vivo was observed in animals that received intratumoral injections 2 h before photoradiation compared to animals injected intraperitoneally at either 2 or 24 h before photoradiation. The treatment protocols were compared with control groups, consisting of Photofrin II administration intratumorally or intraperitoneally without photoradiation, or photoradiation in the absence of Photofrin II. These data indicate that the intratumoral injection regimen with Photofrin II enhanced the efficacy of photodynamic therapy. The greater delay in tumour growth observed after intratumoral administration of Photofrin II suggests a mechanism favouring direct cell damage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellnier D. A., Dougherty T. J. Haematoporphyrin derivative photosensitization and gamma-radiation damage interaction in Chinese hamster ovary fibroblasts. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Oct;50(4):659–664. doi: 10.1080/09553008614551061. [DOI] [PubMed] [Google Scholar]
- Ceckler T. L., Bryant R. G., Penney D. P., Gibson S. L., Hilf R. 31P-NMR spectroscopy demonstrates decreased ATP levels in vivo as an early response to photodynamic therapy. Biochem Biophys Res Commun. 1986 Oct 15;140(1):273–279. doi: 10.1016/0006-291x(86)91086-7. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Studies on the structure of porphyrins contained in Photofrin II. Photochem Photobiol. 1987 Nov;46(5):569–573. doi: 10.1111/j.1751-1097.1987.tb04815.x. [DOI] [PubMed] [Google Scholar]
- Edell E. S., Cortese D. A. Combined effects of hematoporphyrin derivative phototherapy and adriamycin in a murine tumor model. Lasers Surg Med. 1988;8(4):413–417. doi: 10.1002/lsm.1900080413. [DOI] [PubMed] [Google Scholar]
- Gibson S. L., Cohen H. J., Hilf R. Evidence against the production of superoxide by photoirradiation of hematoporphyrin derivative. Photochem Photobiol. 1984 Oct;40(4):441–448. doi: 10.1111/j.1751-1097.1984.tb04615.x. [DOI] [PubMed] [Google Scholar]
- Gibson S. L., Hilf R. Photosensitization of mitochondrial cytochrome c oxidase by hematoporphyrin derivative and related porphyrins in vitro and in vivo. Cancer Res. 1983 Sep;43(9):4191–4197. [PubMed] [Google Scholar]
- Gibson S. L., Murant R. S., Chazen M. D., Kelly M. E., Hilf R. In vitro photosensitization of tumour cell enzymes by photofrin II administered in vivo. Br J Cancer. 1989 Jan;59(1):47–53. doi: 10.1038/bjc.1989.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomer C. J., Dougherty T. J. Determination of [3H]- and [14C]hematoporphyrin derivative distribution in malignant and normal tissue. Cancer Res. 1979 Jan;39(1):146–151. [PubMed] [Google Scholar]
- Gonzalez S., Arnfield M. R., Meeker B. E., Tulip J., Lakey W. H., Chapman J. D., McPhee M. S. Treatment of Dunning R3327-AT rat prostate tumors with photodynamic therapy in combination with misonidazole. Cancer Res. 1986 Jun;46(6):2858–2862. [PubMed] [Google Scholar]
- HILF R., MICHEL I., BELL C., FREEMAN J. J., BORMAN A. BIOCHEMICAL AND MORPHOLOGIC PROPERTIES OF A NEW LACTATING MAMMARY TUMOR LINE IN THE RAT. Cancer Res. 1965 Apr;25:286–299. [PubMed] [Google Scholar]
- Hilf R., Gibson S. L., Penney D. P., Ceckler T. L., Bryant R. G. Early biochemical responses to photodynamic therapy monitored by NMR spectroscopy. Photochem Photobiol. 1987 Nov;46(5):809–817. doi: 10.1111/j.1751-1097.1987.tb04852.x. [DOI] [PubMed] [Google Scholar]
- Hilf R., Murant R. S., Narayanan U., Gibson S. L. Relationship of mitochondrial function and cellular adenosine triphosphate levels to hematoporphyrin derivative-induced photosensitization in R3230AC mammary tumors. Cancer Res. 1986 Jan;46(1):211–217. [PubMed] [Google Scholar]
- Kessel D. Sites of photosensitization by derivatives of hematoporphyrin. Photochem Photobiol. 1986 Oct;44(4):489–493. doi: 10.1111/j.1751-1097.1986.tb04697.x. [DOI] [PubMed] [Google Scholar]
- Kessel D., Thompson P., Musselman B., Chang C. K. Chemistry of hematoporphyrin-derived photosensitizers. Photochem Photobiol. 1987 Nov;46(5):563–568. doi: 10.1111/j.1751-1097.1987.tb04814.x. [DOI] [PubMed] [Google Scholar]
- Kostron H., Bellnier D. A., Lin C. W., Swartz M. R., Martuza R. L. Distribution, retention, and phototoxicity of hematoporphyrin derivative in a rat glioma. Intraneoplastic versus intraperitoneal injection. J Neurosurg. 1986 May;64(5):768–774. doi: 10.3171/jns.1986.64.5.0768. [DOI] [PubMed] [Google Scholar]
- Lambert C. R., Reddi E., Spikes J. D., Rodgers M. A., Jori G. The effects of porphyrin structure and aggregation state on photosensitized processes in aqueous and micellar media. Photochem Photobiol. 1986 Nov;44(5):595–601. doi: 10.1111/j.1751-1097.1986.tb04714.x. [DOI] [PubMed] [Google Scholar]
- Lin C. W., Amano T., Rutledge A. R., Shulok J. R., Prout G. R., Jr Photodynamic effect in an experimental bladder tumor treated with intratumor injection of hematoporphyrin derivative. Cancer Res. 1988 Nov 1;48(21):6115–6120. [PubMed] [Google Scholar]
- McCaughan J. S., Jr, Hawley P. C., Bethel B. H., Walker J. Photodynamic therapy of endobronchial malignancies. Cancer. 1988 Aug 15;62(4):691–701. doi: 10.1002/1097-0142(19880815)62:4<691::aid-cncr2820620408>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Nseyo U. O., Dougherty T. J., Sullivan L. Photodynamic therapy in the management of resistant lower urinary tract carcinoma. Cancer. 1987 Dec 15;60(12):3113–3119. doi: 10.1002/1097-0142(19871215)60:12<3113::aid-cncr2820601242>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Steichen J. D., Dashner K., Martuza R. L. Distribution of hematoporphyrin derivative in canine glioma following intraneoplastic and intraperitoneal injection. J Neurosurg. 1986 Sep;65(3):364–369. doi: 10.3171/jns.1986.65.3.0364. [DOI] [PubMed] [Google Scholar]
- Waldow S. M., Dougherty T. J. Interaction of hyperthermia and photoradiation therapy. Radiat Res. 1984 Feb;97(2):380–385. [PubMed] [Google Scholar]
- Waldow S. M., Henderson B. W., Dougherty T. J. Hyperthermic potentiation of photodynamic therapy employing Photofrin I and II: comparison of results using three animal tumor models. Lasers Surg Med. 1987;7(1):12–22. doi: 10.1002/lsm.1900070104. [DOI] [PubMed] [Google Scholar]
- Winther J., Overgaard J., Ehlers N. The effect of photodynamic therapy alone and in combination with misonidazole or X-rays for management of a retinoblastoma-like tumour. Photochem Photobiol. 1988 Mar;47(3):419–423. doi: 10.1111/j.1751-1097.1988.tb02746.x. [DOI] [PubMed] [Google Scholar]
- Zhou C. N., Milanesi C., Jori G. An ultrastructural comparative evaluation of tumors photosensitized by porphyrins administered in aqueous solution, bound to liposomes or to lipoproteins. Photochem Photobiol. 1988 Oct;48(4):487–492. doi: 10.1111/j.1751-1097.1988.tb02850.x. [DOI] [PubMed] [Google Scholar]


