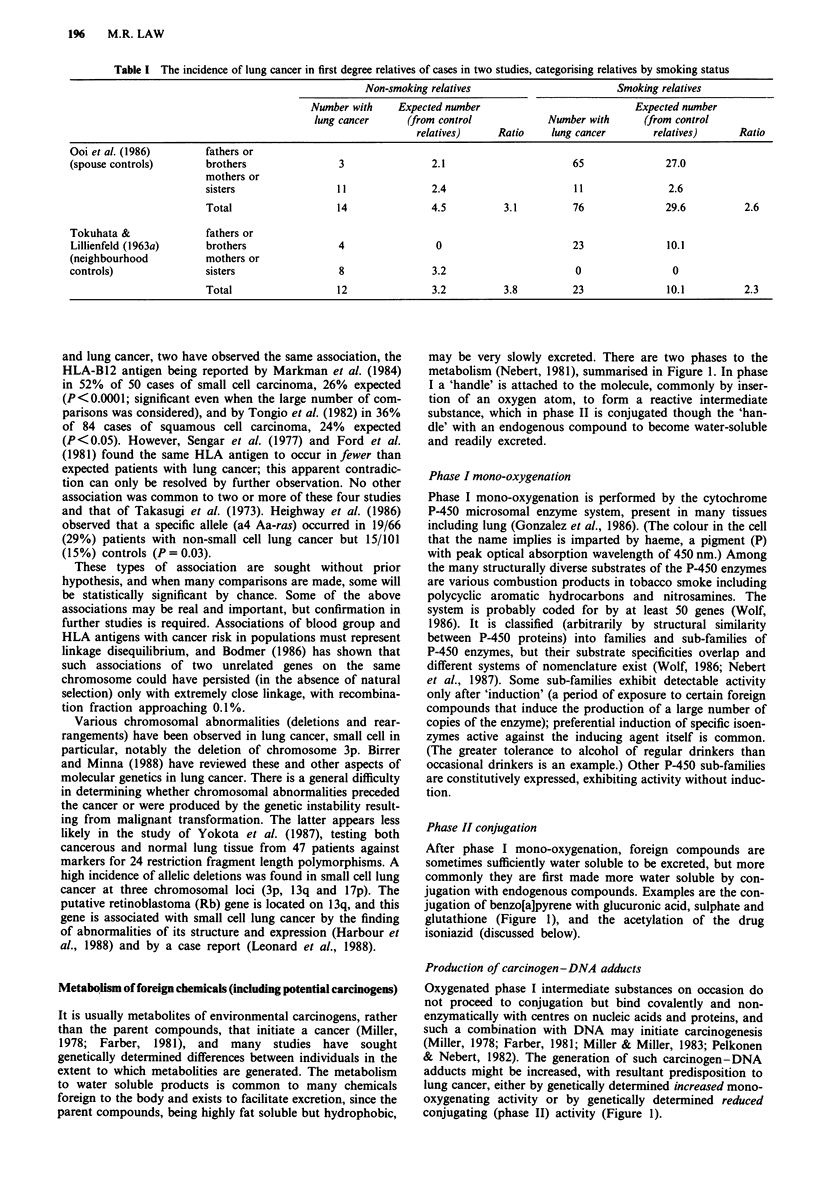
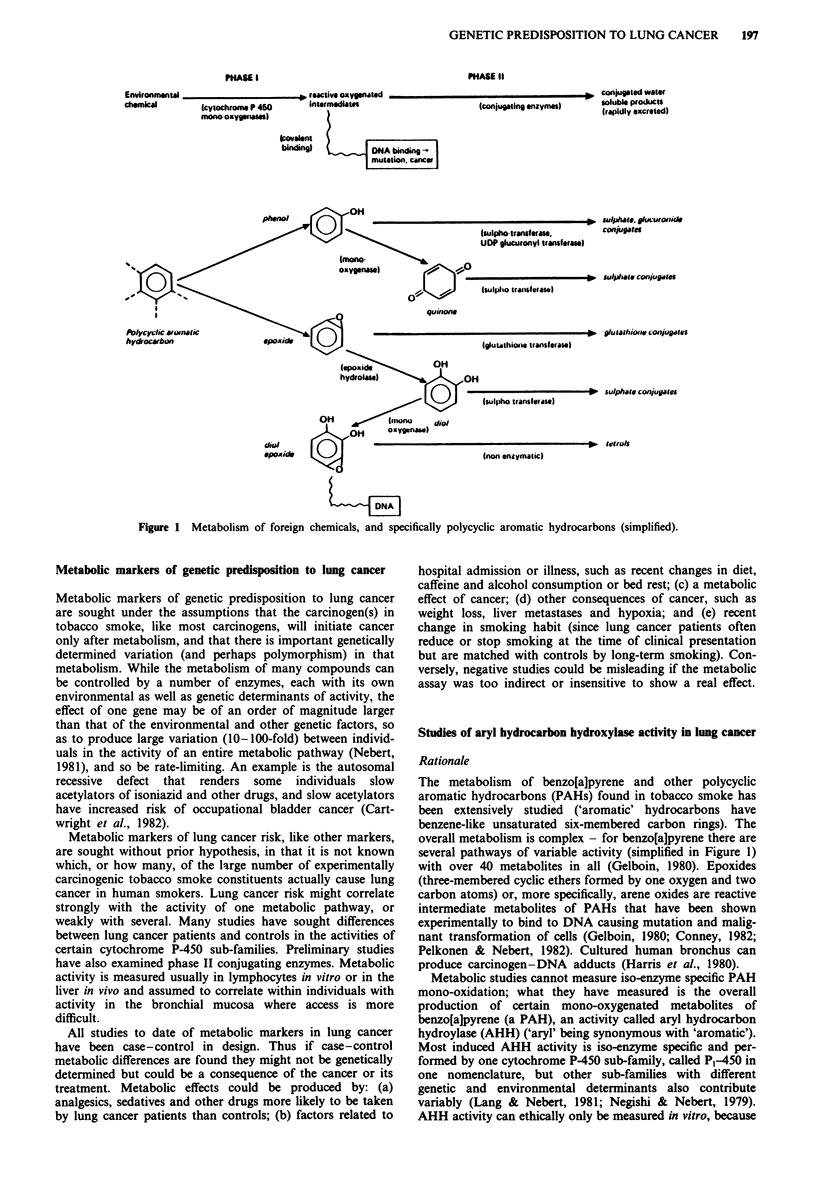
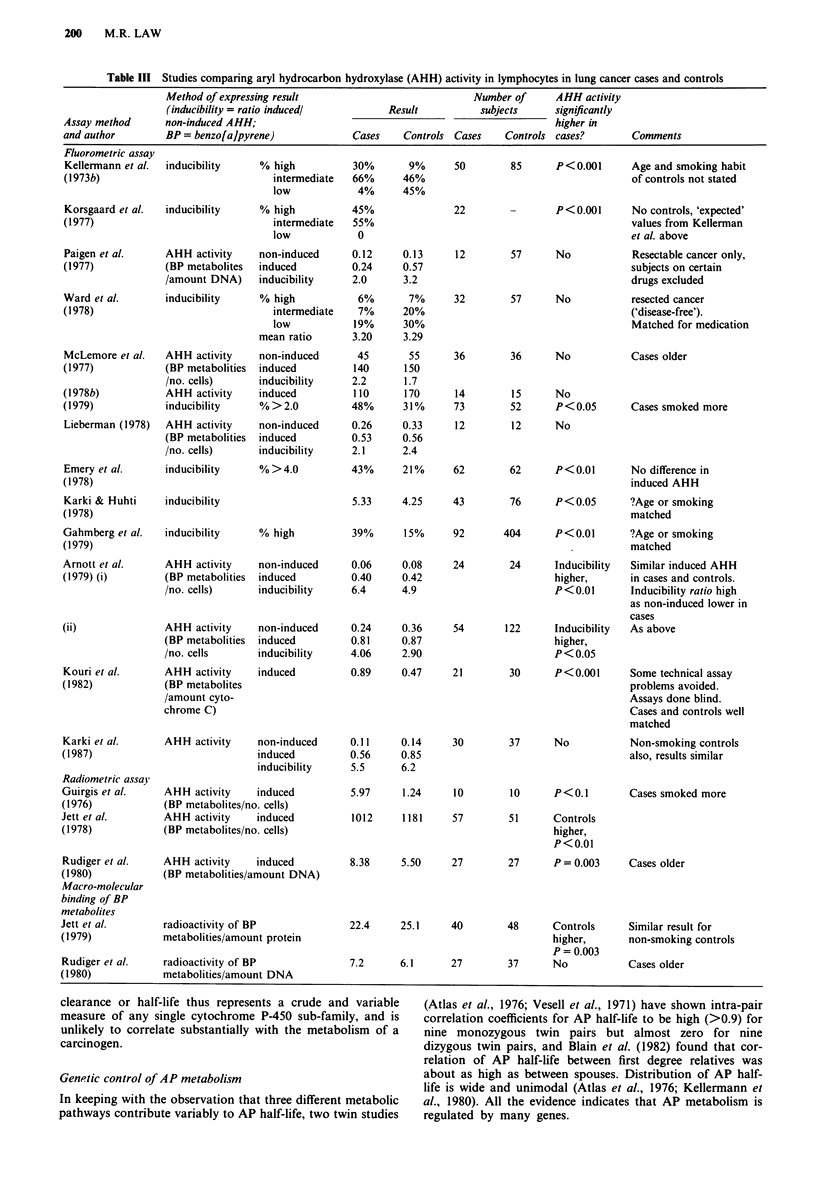
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AIRD I., BENTALL H. H., MEHIGAN J. A., ROBERTS J. A. F. The blood groups in relation to peptic ulceration and carcinoma of colon, rectum, breast, and bronchus; an association between the ABO groups and peptic ulceration. Br Med J. 1954 Aug 7;2(4883):315–321. doi: 10.1136/bmj.2.4883.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambre J., Graeff D., Bures F., Haupt D., Deason K. Antipyrine metabolism and bronchogenic carcinoma. J Med. 1977;8(1):57–70. [PubMed] [Google Scholar]
- Amsbaugh S. C., Ding J. H., Swan D. C., Popescu N. C., Chen Y. T. Expression and chromosomal localization of the cytochrome P1-450 gene in human mitogen-stimulated lymphocytes. Cancer Res. 1986 May;46(5):2423–2427. [PubMed] [Google Scholar]
- Ashley D. J. Blood groups and lung cancer. J Med Genet. 1969 Jun;6(2):183–186. doi: 10.1136/jmg.6.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas S. A., Vesell E. S., Nebert D. W. Genetic control of interindividual variations in the inducibility of aryl hydrocarbon hydroxylase in cultured human lymphocytes. Cancer Res. 1976 Dec;36(12):4619–4630. [PubMed] [Google Scholar]
- Ayesh R., Idle J. R., Ritchie J. C., Crothers M. J., Hetzel M. R. Metabolic oxidation phenotypes as markers for susceptibility to lung cancer. Nature. 1984 Nov 8;312(5990):169–170. doi: 10.1038/312169a0. [DOI] [PubMed] [Google Scholar]
- Beckman G., Eklund A., Fröhlander N., Stjernberg N. Haptoglobin groups and lung cancer. Hum Hered. 1986;36(4):258–260. doi: 10.1159/000153638. [DOI] [PubMed] [Google Scholar]
- Birrer M. J., Minna J. D. Molecular genetics of lung cancer. Semin Oncol. 1988 Jun;15(3):226–235. [PubMed] [Google Scholar]
- Blain P. G., Mucklow J. C., Wood P., Roberts D. F., Rawlins M. D. Family study of antipyrine clearance. Br Med J (Clin Res Ed) 1982 Jan 16;284(6310):150–152. doi: 10.1136/bmj.284.6310.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boobis A. R., Brodie M. J., Kahn G. C., Toverud E. L., Blair I. A., Murray S., Davies D. S. Comparison of the in vivo and in vitro rates of formation of the three main oxidative metabolites of antipyrine in man. Br J Clin Pharmacol. 1981 Dec;12(6):771–777. doi: 10.1111/j.1365-2125.1981.tb01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch R. A., Herbert C. M., Read A. E. Determinants of serum antipyrine half-lives in patients with liver disease. Gut. 1973 Jul;14(7):569–573. doi: 10.1136/gut.14.7.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell E., Busbee D., Warr G., Martin R., Anderson M. D. Induction of aryl hydrocarbon hydroxylase in human lymphocytes and pulmonary alveolar macrophages--a comparison. Life Sci. 1973 Dec 16;13(12):1649–1654. doi: 10.1016/0024-3205(73)90112-4. [DOI] [PubMed] [Google Scholar]
- Caporaso N., Hayes R. B., Dosemeci M., Hoover R., Ayesh R., Hetzel M., Idle J. Lung cancer risk, occupational exposure, and the debrisoquine metabolic phenotype. Cancer Res. 1989 Jul 1;49(13):3675–3679. [PubMed] [Google Scholar]
- Cartwright R. A., Glashan R. W., Rogers H. J., Ahmad R. A., Barham-Hall D., Higgins E., Kahn M. A. Role of N-acetyltransferase phenotypes in bladder carcinogenesis: a pharmacogenetic epidemiological approach to bladder cancer. Lancet. 1982 Oct 16;2(8303):842–845. doi: 10.1016/s0140-6736(82)90810-8. [DOI] [PubMed] [Google Scholar]
- Cobb R. R., Stoming T. A., Whitney J. B., 3rd The aryl hydrocarbon hydroxylase (Ah) locus and a novel restriction-fragment length polymorphism (RFLP) are located on mouse chromosome 12. Biochem Genet. 1987 Jun;25(5-6):401–413. doi: 10.1007/BF00554549. [DOI] [PubMed] [Google Scholar]
- Cohen G. M., Mehta R., Meredith-Brown M. Large interindividual variations in metabolism of benzo(alpha)pyrene by peripheral lung tissue from lung cancer patients. Int J Cancer. 1979 Aug;24(2):129–133. doi: 10.1002/ijc.2910240202. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982 Dec;42(12):4875–4917. [PubMed] [Google Scholar]
- Danhof M., Breimer D. D. Studies on the different metabolic pathways of antipyrine in man. I. Oral administration of 250, 500 and 1000 mg to healthy volunteers. Br J Clin Pharmacol. 1979 Dec;8(6):529–537. doi: 10.1111/j.1365-2125.1979.tb01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day N., Holmes L. B. The incidence of genetic disease in a University hospital population. Am J Hum Genet. 1973 May;25(3):237–246. [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum M., Baur M. P., Dengler H. J., Osikowska-Evers B. O., Tieves G., Zekorn C., Rittner C. Chromosomal assignment of human cytochrome P-450 (debrisoquine/sparteine type) to chromosome 22. Br J Clin Pharmacol. 1987 Apr;23(4):455–458. doi: 10.1111/j.1365-2125.1987.tb03075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum M. Polymorphic drug oxidation in humans. Fed Proc. 1984 May 15;43(8):2298–2302. [PubMed] [Google Scholar]
- Emery A. E., Anand R., Danford N., Duncan W., Paton L. Aryl-hydrocarbon-hydroxylase inducibility in patients with cancer. Lancet. 1978 Mar 4;1(8062):470–472. doi: 10.1016/s0140-6736(78)90135-6. [DOI] [PubMed] [Google Scholar]
- Evans D. A., Mahgoub A., Sloan T. P., Idle J. R., Smith R. L. A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. J Med Genet. 1980 Apr;17(2):102–105. doi: 10.1136/jmg.17.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E. Chemical carcinogenesis. N Engl J Med. 1981 Dec 3;305(23):1379–1389. doi: 10.1056/NEJM198112033052304. [DOI] [PubMed] [Google Scholar]
- Ford C. H., Newman C. E., Mackintosh P. HLA frequency and prognosis in lung cancer. Br J Cancer. 1981 May;43(5):610–614. doi: 10.1038/bjc.1981.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Sekki A., Kosunen T. U., Holsti L. R., Mäkelä O. Induction of aryl hydrocarbon hydroxylase activity and pulmonary carcinoma. Int J Cancer. 1979 Mar 15;23(3):302–305. doi: 10.1002/ijc.2910230305. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980 Oct;60(4):1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Jaiswal A. K., Nebert D. W. P450 genes: evolution, regulation, and relationship to human cancer and pharmacogenetics. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):879–890. doi: 10.1101/sqb.1986.051.01.101. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Skoda R. C., Kimura S., Umeno M., Zanger U. M., Nebert D. W., Gelboin H. V., Hardwick J. P., Meyer U. A. Characterization of the common genetic defect in humans deficient in debrisoquine metabolism. Nature. 1988 Feb 4;331(6155):442–446. doi: 10.1038/331442a0. [DOI] [PubMed] [Google Scholar]
- Guirgis H. A., Lynch H. T., Mate T., Harris R. E., Wells I., Caha L., Anderson J., Maloney K., Rankin L. Aryl-hydrocarbon hydroxylase activity in lymphocytes from lung cancer patients and normal controls. Oncology. 1976;33(3):105–109. doi: 10.1159/000225116. [DOI] [PubMed] [Google Scholar]
- Gurtoo H. L., Minowada J., Paigen B., Parker N. B., Hayner N. T. Factors influencing the measurement and the reproducibility of aryl hydrocarbon hydroxylase activity in culutred human lymphocytes. J Natl Cancer Inst. 1977 Sep;59(3):787–798. doi: 10.1093/jnci/59.3.787. [DOI] [PubMed] [Google Scholar]
- Hankinson O., Andersen R. D., Birren B. W., Sander F., Negishi M., Nebert D. W. Mutations affecting the regulation of transcription of the cytochrome P1-450 gene in the mouse Hepa-1 cell line. J Biol Chem. 1985 Feb 10;260(3):1790–1795. [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. C., Autrup H., Connor R., Barrett L. A., McDowell E. M., Trump B. F. Interindividual variation in binding of benzo[a]pyrene to DNA in cultured human bronchi. Science. 1976 Dec 3;194(4269):1067–1069. doi: 10.1126/science.982061. [DOI] [PubMed] [Google Scholar]
- Harris C. C., Autrup H., Stoner G., Yang S. K., Leutz J. C., Gelboin H. V., Selkirk J. K., Connor R. J., Barrett L. A., Jones R. T. Metabolism of benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in cultured human bronchus and pancreatic duct. Cancer Res. 1977 Sep;37(9):3349–3355. [PubMed] [Google Scholar]
- Harris C. C., Mulvihill J. J., Thorgeirsson S. S., Minna J. D. Individual differences in cancer susceptibility. Ann Intern Med. 1980 Jun;92(6):809–825. doi: 10.7326/0003-4819-92-6-809. [DOI] [PubMed] [Google Scholar]
- Hart P., Farrell G. C., Cooksley W. G., Powell L. W. Enhanced drug metabolism in cigarette smokers. Br Med J. 1976 Jul 17;2(6028):147–149. doi: 10.1136/bmj.2.6028.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heighway J., Thatcher N., Cerny T., Hasleton P. S. Genetic predisposition to human lung cancer. Br J Cancer. 1986 Apr;53(4):453–457. doi: 10.1038/bjc.1986.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C. E., Gonzalez F. J., Kozak C. A., Nebert D. W. Regional linkage analysis of the dioxin-inducible P-450 gene family on mouse chromosome 9. Biochem Biophys Res Commun. 1985 Jul 16;130(1):396–406. doi: 10.1016/0006-291x(85)90430-9. [DOI] [PubMed] [Google Scholar]
- Hutter R. V. Cancer prevention and detection. Status report and future prospects. Cancer. 1988 Jun 1;61(11 Suppl):2372–2378. doi: 10.1002/1097-0142(19880601)61:11+<2372::aid-cncr2820611304>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Idle J. R., Mahgoub A., Angelo M. M., Dring L. G., Lancaster R., Smith R. L. The metabolism of [14C]-debrisoquine in man. Br J Clin Pharmacol. 1979 Mar;7(3):257–266. doi: 10.1111/j.1365-2125.1979.tb00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel D. I., Whitlock J. P., Jr Regulation of cytochrome P1-450 gene transcription by 2,3,7, 8-tetrachlorodibenzo-p-dioxin in wild type and variant mouse hepatoma cells. J Biol Chem. 1984 May 10;259(9):5400–5402. [PubMed] [Google Scholar]
- Jaiswal A. K., Gonzalez F. J., Nebert D. W. Human P1-450 gene sequence and correlation of mRNA with genetic differences in benzo[a]pyrene metabolism. Nucleic Acids Res. 1985 Jun 25;13(12):4503–4520. doi: 10.1093/nar/13.12.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal A. K., Nebert D. W., McBride O. W., Gonzalez F. J. Human P(3)450: cDNA and complete protein sequence, repetitive Alu sequences in the 3' nontranslated region, and localization of gene to chromosome 15. J Exp Pathol. 1987 Winter;3(1):1–17. [PubMed] [Google Scholar]
- Jakoubková J., Májský A. Blood groups and neoplastic disease. Neoplasma. 1965;12(6):611–616. [PubMed] [Google Scholar]
- Jett J. R., Branum E. L., Fontana R. S., Taylor W. F., Moses H. L. Macromolecular binding of 3H-benzo(a)pyrene metabolites and lymphocyte transformation in patients with lung cancer, and in smoking and nonsmoking control subjects. Am Rev Respir Dis. 1979 Aug;120(2):369–375. doi: 10.1164/arrd.1979.120.2.369. [DOI] [PubMed] [Google Scholar]
- Jett J. R., Moses H. L., Branum E. L., Taylor W. F., Fontana R. S. Benzo(a)pyrene metabolism and blast transformation in peripheral blood mononuclear cells from smoking and nonsmoking populations and lung cancer patients. Cancer. 1978 Jan;41(1):192–200. doi: 10.1002/1097-0142(197801)41:1<192::aid-cncr2820410128>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Joishy S. K., Cooper R. A., Rowley P. T. Alveolar cell carcinoma in identical twins. Similarity in time of onset, histochemistry, and site of metastasis. Ann Intern Med. 1977 Oct;87(4):447–450. doi: 10.7326/0003-4819-87-4-447. [DOI] [PubMed] [Google Scholar]
- Kalamegham R., Krishnaswamy K., Krishnamurthy S., Bhargava R. N. Metabolism of drugs and carcinogens in man: antipyrine elimination as an indicator. Clin Pharmacol Ther. 1979 Jan;25(1):67–73. doi: 10.1002/cpt197925167. [DOI] [PubMed] [Google Scholar]
- Kapitulnik J., Popper P. J., Conney A. H. Comparative metabolism of benzo[a]pyrene and drugs in human liver. Clin Pharmacol Ther. 1977 Feb;21(2):166–176. doi: 10.1002/cpt1977212166. [DOI] [PubMed] [Google Scholar]
- Kato R., Takanaka A., Takahashi A., Onoda K. Drug metabolism in tumor-bearing rats. I. Activities of NADPH-linked electron transport and drug-metabolizing enzyme systems in liver microsomes of tumor-bearing rats. Jpn J Pharmacol. 1968 Jun;18(2):224–244. [PubMed] [Google Scholar]
- Kellermann G., Jett J. R., Luyten-Kellermann M., Moses H. L., Fontana R. S. Variation of microsomal mixed function oxidase(s) and human lung cancer. Cancer. 1980 Mar 15;45(6):1438–1442. doi: 10.1002/1097-0142(19800315)45:6<1438::aid-cncr2820450623>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kellermann G., Shaw C. R., Luyten-Kellerman M. Aryl hydrocarbon hydroxylase inducibility and bronchogenic carcinoma. N Engl J Med. 1973 Nov 1;289(18):934–937. doi: 10.1056/NEJM197311012891802. [DOI] [PubMed] [Google Scholar]
- Korsgaard R., Trell E. Aryl hydrocarbon hydroxylase and bronchogenic carcinomas associated with smoking. Lancet. 1978 May 20;1(8073):1103–1104. doi: 10.1016/s0140-6736(78)90952-2. [DOI] [PubMed] [Google Scholar]
- Kouri R. E., Billups L. H., Rude T. H., Whitmire C. E., Sass B., Henry C. J. Correlation of inducibility of aryl hydrocarbon hydroxylase with susceptibility to 3-methylcholanthrene-induced lung cancers. Cancer Lett. 1980 Jun;9(4):277–284. doi: 10.1016/0304-3835(80)90018-x. [DOI] [PubMed] [Google Scholar]
- Kouri R. E., McKinney C. E., Slomiany D. J., Snodgrass D. R., Wray N. P., McLemore T. L. Positive correlation between high aryl hydrocarbon hydroxylase activity and primary lung cancer as analyzed in cryopreserved lymphocytes. Cancer Res. 1982 Dec;42(12):5030–5037. [PubMed] [Google Scholar]
- Kärki N. T., Pokela R., Nuutinen L., Pelkonen O. Aryl hydrocarbon hydroxylase in lymphocytes and lung tissue from lung cancer patients and controls. Int J Cancer. 1987 May 15;39(5):565–570. doi: 10.1002/ijc.2910390505. [DOI] [PubMed] [Google Scholar]
- Lang M. A., Nebert D. W. Structural gene products of the Ah locus. Evidence for many unique P-450-mediated monooxygenase activities reconstituted from 3-methylcholanthrene-treated C57BL/6N mouse liver microsomes. J Biol Chem. 1981 Dec 10;256(23):12058–12067. [PubMed] [Google Scholar]
- Law M. R., Hetzel M. R., Idel J. R. Debrisoquine metabolism and genetic predisposition to lung cancer. Br J Cancer. 1989 May;59(5):686–687. doi: 10.1038/bjc.1989.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J. Aryl hydrocarbon hydroxylase in bronchogenic carcinoma. N Engl J Med. 1978 Mar 23;298(12):686–687. doi: 10.1056/NEJM197803232981213. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Fain P. R., Albano W. A., Ruma T., Black L., Lynch J., Shonka M. Genetic/epidemiological findings in a study of smoking-associated tumors. Cancer Genet Cytogenet. 1982 Jun;6(2):163–169. doi: 10.1016/0165-4608(82)90081-4. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Kimberling W. J., Markvicka S. E., Biscone K. A., Lynch J. F., Whorton E., Jr, Mailliard J. Genetics and smoking-associated cancers. A study of 485 families. Cancer. 1986 Apr 15;57(8):1640–1646. doi: 10.1002/1097-0142(19860415)57:8<1640::aid-cncr2820570833>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Markman M., Braine H. G., Abeloff M. D. Histocompatibility antigens in small cell carcinoma of the lung. Cancer. 1984 Dec 15;54(12):2943–2945. doi: 10.1002/1097-0142(19841215)54:12<2943::aid-cncr2820541221>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- McLemore T. L., Martin R. R., Busbee D. L., Richie R. C., Springer R. R., Toppell K. L., Cantrell E. T. Aryl hydrocarbon hydroxylase activity in pulmonary macrophages and lymphocytes from lung cancer and noncancer patients. Cancer Res. 1977 Apr;37(4):1175–1181. [PubMed] [Google Scholar]
- McLemore T. L., Martin R. R., Pickard L. R., Springer R. R., Wray N. P., Toppell K. L., Mattox K. L., Guinn G. A., Cantrell E. T., Busbee D. L. Analysis of aryl hydrocarbon hydroxylase activity in human lung tissue, pulmonary macrophages, and blood lymphocytes. Cancer. 1978 Jun;41(6):2292–2300. doi: 10.1002/1097-0142(197806)41:6<2292::aid-cncr2820410630>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- McLemore T. L., Martin R. R., Springer R. R., Wray N., Cantrell E. T., Busbee D. L. Aryl hydrocarbon hydroxylase activity in pulmonary alveolar macrophages and lymphocytes from lung cancer and noncancer patients: a correlation with family histories of cancer. Biochem Genet. 1979 Oct;17(9-10):795–806. doi: 10.1007/BF00504304. [DOI] [PubMed] [Google Scholar]
- McLemore T. L., Martin R. R., Wray N. P., Cantrell E. T., Busbee D. L. Dissociation between aryl hydrocarbon hydroxylase activity in cultured pulmonary macrophages and blood lymphocytes from lung cancer patients. Cancer Res. 1978 Nov;38(11 Pt 1):3805–3811. [PubMed] [Google Scholar]
- Meehan R. R., Speed R. M., Gosden J. R., Rout D., Hutton J. J., Taylor B. A., Hilkens J., Kroezen V., Hilgers J., Adesnik M. Chromosomal organization of the cytochrome P450-2C gene family in the mouse: a locus associated with constitutive aryl hydrocarbon hydroxylase. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2662–2666. doi: 10.1073/pnas.85.8.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. C. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978 Jun;38(6):1479–1496. [PubMed] [Google Scholar]
- Miller J. A., Miller E. C. The metabolic activation and nucleic acid adducts of naturally-occurring carcinogens: recent results with ethyl carbamate and the spice flavors safrole and estragole. Br J Cancer. 1983 Jul;48(1):1–15. doi: 10.1038/bjc.1983.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W. Clinical pharmacology. Possible clinical importance of genetic differences in drug metabolism. Br Med J (Clin Res Ed) 1981 Aug 22;283(6290):537–542. doi: 10.1136/bmj.283.6290.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Goujon F. M., Gielen J. E. Aryl hydrocarbon hydroxylase induction by polycyclic hydrocarbons: simple autosomal dominant trait in the mouse. Nat New Biol. 1972 Mar 29;236(65):107–110. doi: 10.1038/newbio236107a0. [DOI] [PubMed] [Google Scholar]
- Negishi M., Nebert D. W. Structural gene products of the Ah locus. Genetic and immunochemical evidence for two forms of mouse liver cytochrome P-450 induced by 3-methylcholanthrene. J Biol Chem. 1979 Nov 10;254(21):11015–11023. [PubMed] [Google Scholar]
- O'Malley K., Crooks J., Duke E., Stevenson I. H. Effect of age and sex on human drug metabolism. Br Med J. 1971 Sep 11;3(5775):607–609. doi: 10.1136/bmj.3.5775.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocraft K. P., Muskett J. M., Brown S. Localization of the human aryl hydrocarbon hydroxylase gene to the 2q31----2pter region of chromosome 2. Ann Hum Genet. 1985 Jul;49(Pt 3):237–239. doi: 10.1111/j.1469-1809.1985.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Oesch F., Schmassmann H., Ohnhaus E., Althaus U., Lorenz J. Monooxygenase, epoxide hydrolase, and glutathione-S-transferase activities in human lung. Variation between groups of bronchogenic carcinoma and non-cancer patients and interindividual differences. Carcinogenesis. 1980;1(10):827–835. doi: 10.1093/carcin/1.10.827. [DOI] [PubMed] [Google Scholar]
- Okey A. B., Bondy G. P., Mason M. E., Nebert D. W., Forster-Gibson C. J., Muncan J., Dufresne M. J. Temperature-dependent cytosol-to-nucleus translocation of the Ah receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin in continuous cell culture lines. J Biol Chem. 1980 Dec 10;255(23):11415–11422. [PubMed] [Google Scholar]
- Okuda T., Vesell E. S., Plotkin E., Tarone R., Bast R. C., Gelboin H. V. Interindividual and intraindividual variations in aryl hydrocarbon hydroxylase in monocytes from monozygotic and dizygotic twins. Cancer Res. 1977 Nov;37(11):3904–3911. [PubMed] [Google Scholar]
- PARKER G., WALSH R. J. Blood groups and disease: carcinoma of various organs. Med J Aust. 1958 Dec 20;45(25):835–837. [PubMed] [Google Scholar]
- Paigen B., Gurtoo H. L., Minowada J., Houten L., Vincent R., Paigen K., Parker N. B., Ward E., Hayner N. T. Questionable relation of aryl hydrocarbon hydroxylase to lung-cancer risk. N Engl J Med. 1977 Aug 18;297(7):346–350. doi: 10.1056/NEJM197708182970702. [DOI] [PubMed] [Google Scholar]
- Paigen B., Ward E., Steenland K., Houten L., Gurtoo H. L., Minowada J. Aryl hydrocarbon hydroxylase in cultured lymphocytes of twins. Am J Hum Genet. 1978 Sep;30(5):561–571. [PMC free article] [PubMed] [Google Scholar]
- Paul S. M., Bacharach B., Goepp C. A genetic influence on alveolar cell carcinoma. J Surg Oncol. 1987 Dec;36(4):249–252. doi: 10.1002/jso.2930360407. [DOI] [PubMed] [Google Scholar]
- Pelkonen O., Nebert D. W. Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol Rev. 1982 Jun;34(2):189–222. [PubMed] [Google Scholar]
- Philip P. A., Fitzgerald D. L., Cartwright R. A., Peake M. D., Rogers H. J. Polymorphic N-acetylation capacity in lung cancer. Carcinogenesis. 1988 Mar;9(3):491–493. doi: 10.1093/carcin/9.3.491. [DOI] [PubMed] [Google Scholar]
- RENNIE H. M., HABER R. W. Blood groups and carcinoma of the lung. Med J Aust. 1961 Jul 8;48(2):61–62. doi: 10.5694/j.1326-5377.1961.tb82571.x. [DOI] [PubMed] [Google Scholar]
- Roberts T. E., Hasleton P., Swindell R., Lawson R. Blood groups and lung cancer. Br J Cancer. 1988 Aug;58(2):278–278. doi: 10.1038/bjc.1988.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso R., Donelli M. G., Franchi G., Garattini S. Impairement of drug metabolism in tumor-bearing animals. Eur J Cancer. 1971 Dec;7(6):565–577. doi: 10.1016/0014-2964(71)90064-8. [DOI] [PubMed] [Google Scholar]
- Rüdiger H. W., Heisig V., Hain E. Enhanced benzo(a)pyrene metabolism and formation of DNA adducts in monocytes of patients with lung cancer. J Cancer Res Clin Oncol. 1980;96(3):295–302. doi: 10.1007/BF00408102. [DOI] [PubMed] [Google Scholar]
- Sabadie N., Richter-Reichhelm H. B., Saracci R., Mohr U., Bartsch H. Inter-individual differences in oxidative benzo(a)pyrene metabolism by normal and tumorous surgical lung specimens from 105 lung cancer patients. Int J Cancer. 1981;27(4):417–425. doi: 10.1002/ijc.2910270402. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Pero R. W., Miller D. G., Beattie E. J. A glutathione transferase in human leukocytes as a marker for the susceptibility to lung cancer. Carcinogenesis. 1986 May;7(5):751–753. doi: 10.1093/carcin/7.5.751. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Pero R. W. The hereditary transmission of high glutathione transferase activity towards trans-stilbene oxide in human mononuclear leukocytes. Hum Genet. 1985;69(1):66–68. doi: 10.1007/BF00295531. [DOI] [PubMed] [Google Scholar]
- Sengar D. P., McLeish W. A., Stewart T. H., Harris J. E. HLA antigens in bronchogenic carcinoma. Oncology. 1977;34(4):143–145. doi: 10.1159/000225208. [DOI] [PubMed] [Google Scholar]
- Shapiro W., Park J., DiBianco R., Singh S. N., Katz R. J., Fletcher R. Comparison of nadolol, a new long-acting beta-receptor blocking agent, and placebo in the treatment of stable angina pectoris. Chest. 1981 Oct;80(4):425–430. doi: 10.1378/chest.80.4.425. [DOI] [PubMed] [Google Scholar]
- Shimada T., Misono K. S., Guengerich F. P. Human liver microsomal cytochrome P-450 mephenytoin 4-hydroxylase, a prototype of genetic polymorphism in oxidative drug metabolism. Purification and characterization of two similar forms involved in the reaction. J Biol Chem. 1986 Jan 15;261(2):909–921. [PubMed] [Google Scholar]
- Skoda R. C., Gonzalez F. J., Demierre A., Meyer U. A. Two mutant alleles of the human cytochrome P-450db1 gene (P450C2D1) associated with genetically deficient metabolism of debrisoquine and other drugs. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5240–5243. doi: 10.1073/pnas.85.14.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan T. P., Lancaster R., Shah R. R., Idle J. R., Smith R. L. Genetically determined oxidation capacity and the disposition of debrisoquine. Br J Clin Pharmacol. 1983 Apr;15(4):443–450. doi: 10.1111/j.1365-2125.1983.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner E., Iselius L., Alván G., Lindsten J., Sjöqvist F. A family study of genetic and environmental factors determining polymorphic hydroxylation of debrisoquin. Clin Pharmacol Ther. 1985 Oct;38(4):394–401. doi: 10.1038/clpt.1985.193. [DOI] [PubMed] [Google Scholar]
- TOKUHATA G. K., LILIENFELD A. M. Familial aggregation of lung cancer among hospital patients. Public Health Rep. 1963 Apr;78:277–283. [PMC free article] [PubMed] [Google Scholar]
- TOKUHATA G. K., LILIENFELD A. M. Familial aggregation of lung cancer in humans. J Natl Cancer Inst. 1963 Feb;30:289–312. [PubMed] [Google Scholar]
- Takasugi M., Terasaki P. I., Henderson B., Mickey M. R., Menck H., Thompson R. W. HL-A antigens in solid tumors. Cancer Res. 1973 Apr;33(4):648–650. [PubMed] [Google Scholar]
- Tongio M. M., Kerschen C., Pauli G., Roeslin N., Grange D., Mayer S. HLA antigens and primary bronchial carcinoma. Cancer. 1982 Jun 15;49(12):2485–2488. doi: 10.1002/1097-0142(19820615)49:12<2485::aid-cncr2820491212>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Trell L., Korsgaard R., Janzon L., Trell E. Distribution and reproducibility of aryl hydrocarbon hydroxylase inducibility in a prospective population study of middle-aged male smokers and nonsmokers. Cancer. 1985 Oct 15;56(8):1988–1994. doi: 10.1002/1097-0142(19851015)56:8<1988::aid-cncr2820560817>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Tschanz C., Hignite C. E., Huffman D. H., Azarnoff D. L. Metabolic disposition of antipyrine in patients with lung cancer. Cancer Res. 1977 Nov;37(11):3881–3886. [PubMed] [Google Scholar]
- Vesell E. S., Page J. G., Passananti G. T. Genetic and environmental factors affecting ethanol metabolism in man. Clin Pharmacol Ther. 1971 Mar-Apr;12(2):192–201. doi: 10.1002/cpt1971122part1192. [DOI] [PubMed] [Google Scholar]
- Vesell E. S. The antipyrine test in clinical pharmacology: conceptions and misconceptions. Clin Pharmacol Ther. 1979 Sep;26(3):275–286. doi: 10.1002/cpt1979263275. [DOI] [PubMed] [Google Scholar]
- Vestal R. E., Norris A. H., Tobin J. D., Cohen B. H., Shock N. W., Andres R. Antipyrine metabolism in man: influence of age, alcohol, caffeine, and smoking. Clin Pharmacol Ther. 1975 Oct;18(4):425–432. doi: 10.1002/cpt1975184425. [DOI] [PubMed] [Google Scholar]
- Ward E., Paigen B., Steenland K., Vincent R., Minowada J., Gurtoo H. L., Sartori P., Havens M. B. Aryl hydrocarbon hydroxylase in persons with lung or laryngeal cancer. Int J Cancer. 1978 Oct 15;22(4):384–389. doi: 10.1002/ijc.2910220404. [DOI] [PubMed] [Google Scholar]
- Wiebel F. J., Hlavica P., Grzeschik K. H. Expression of aromatic polycyclic hydrocarbon-induced monooxygenase (aryl hydrocarbon hydroxylase) in man x mouse hybrids is associated with human chromosome 2. Hum Genet. 1981;59(4):277–280. doi: 10.1007/BF00295458. [DOI] [PubMed] [Google Scholar]
- Wolff T., Distlerath L. M., Worthington M. T., Groopman J. D., Hammons G. J., Kadlubar F. F., Prough R. A., Martin M. V., Guengerich F. P. Substrate specificity of human liver cytochrome P-450 debrisoquine 4-hydroxylase probed using immunochemical inhibition and chemical modeling. Cancer Res. 1985 May;45(5):2116–2122. [PubMed] [Google Scholar]
- Yokota J., Wada M., Shimosato Y., Terada M., Sugimura T. Loss of heterozygosity on chromosomes 3, 13, and 17 in small-cell carcinoma and on chromosome 3 in adenocarcinoma of the lung. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9252–9256. doi: 10.1073/pnas.84.24.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]


