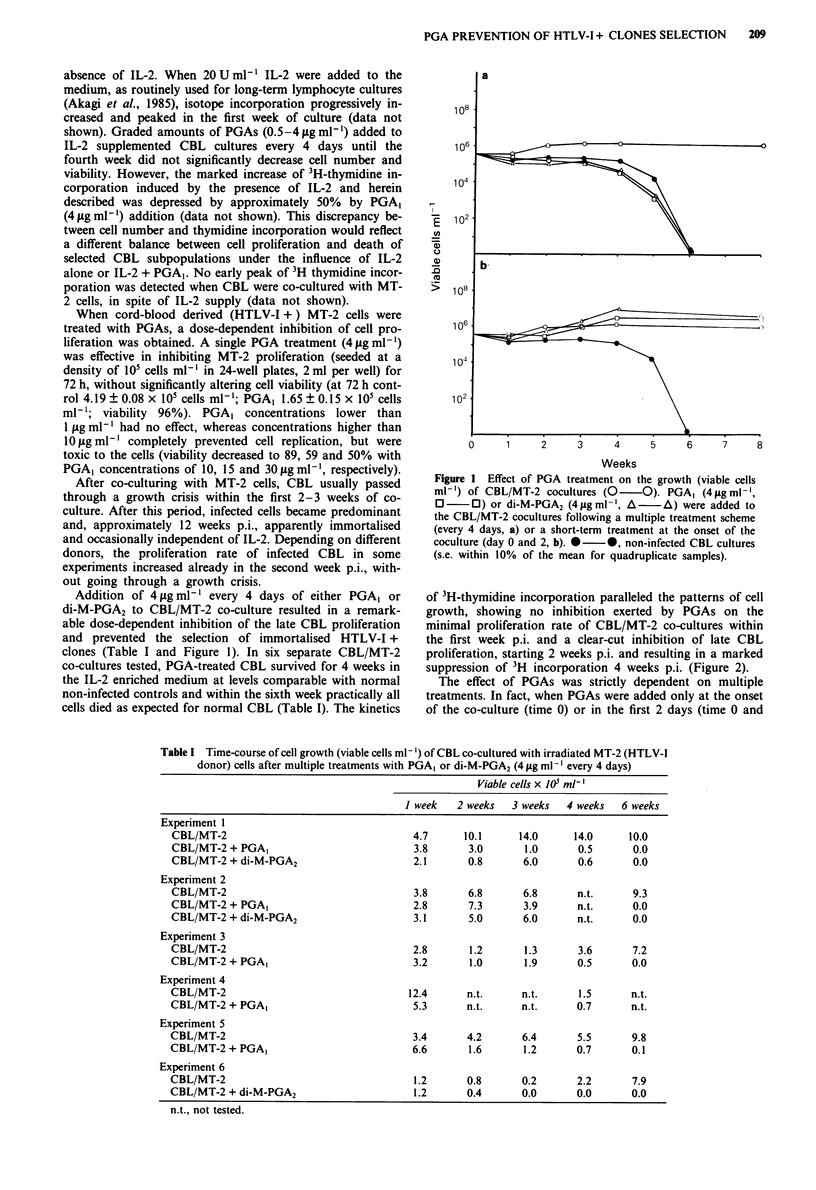
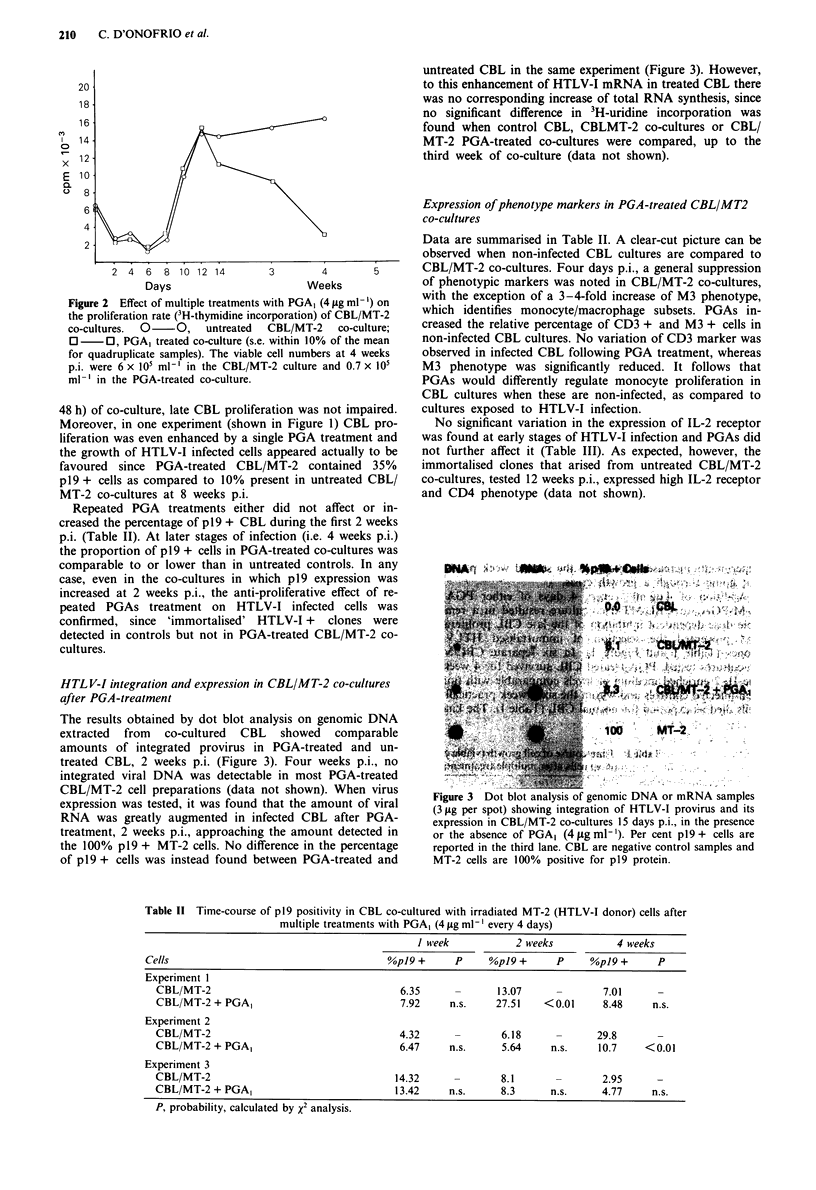
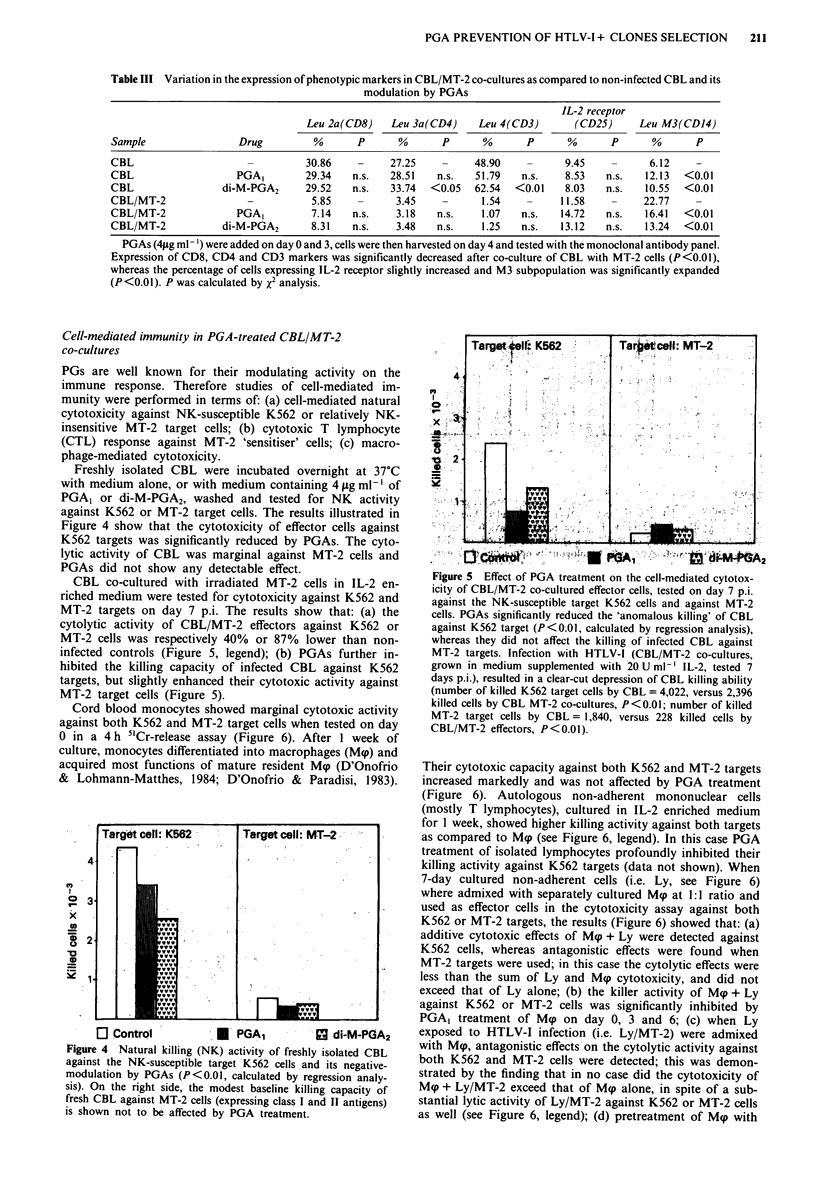
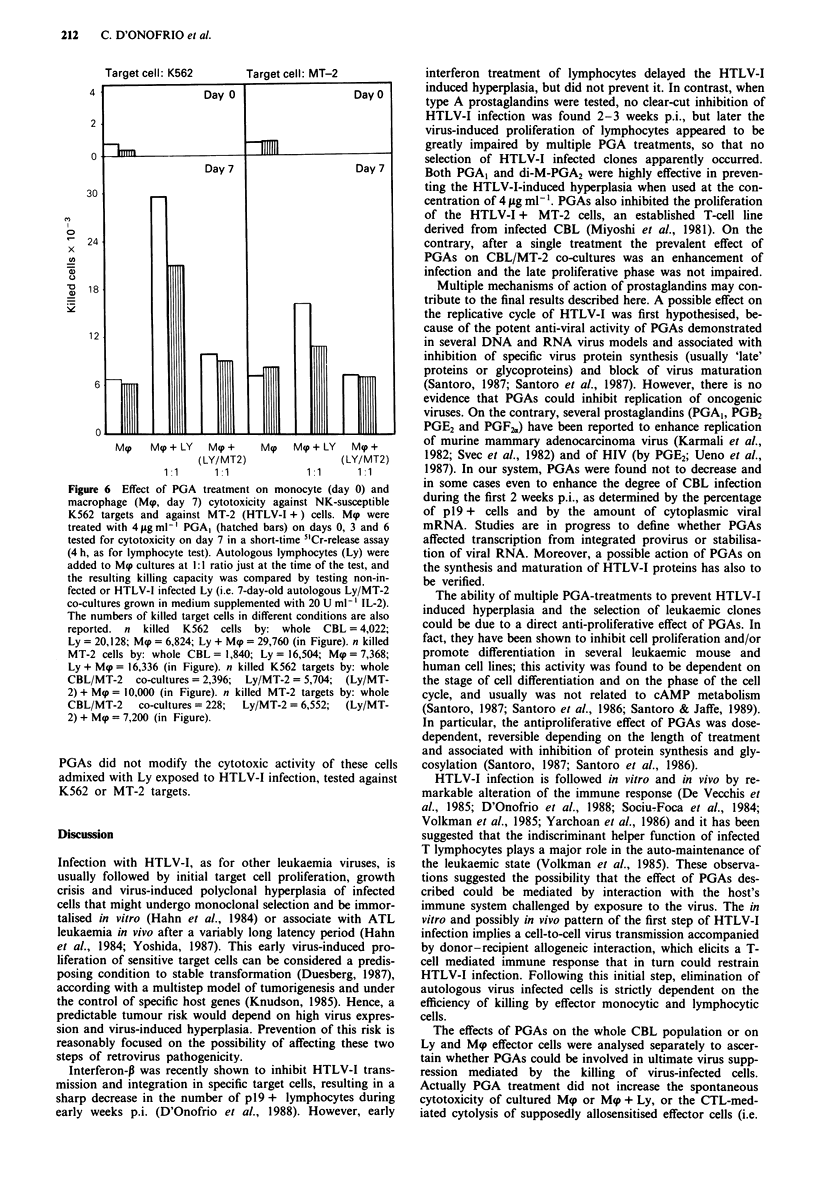
Abstract
Type A prostaglandins (PGA1 and 16,16-dimethyl-PGA2-methyl ester) were found to block the proliferation of HTLV-I infected cord blood lymphocytes (CBL) in vitro, thus preventing the clonal immortalisation that is considered as a predisposing condition to HTLV-I positive leukaemia. PGA1 and di-M-PGA2 did not affect the long-term survival of normal non-infected CBL, whereas they suppressed the proliferation of an established cord-blood derived HTLV-I positive cell line, MT-2. As shown by the number of HTLV-I infected p19+ cells, the block of the selection of immortalised, infected clones by PGAs did not appear to be due to an inhibition of early stages of HTLV-I infection. The possibility that the effect of PGAs could be mediated by an action on the immune response was also examined. PGAs regulated the cell-mediated cytotoxic function of CBL to a different extent when normal non-infected or HTLV-I exposed CBL were compared. In fact, PGAs down-regulated the natural killing and macrophage/lymphocyte cytotoxic response of normal CBL, whereas they did not modify the already depressed immune response of CBL challenged with HTLV-I. These results suggest that the protective effect of PGAs against HTLV-I infection in vitro is mostly related to the direct suppression of the clonal expansion of virus-infected cells, rather than to the anti-viral activity or modulation of the cell-mediated immunity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi T., Ohtsuki Y., Takahashi K., Takeda I., Oka T., Miyoshi I. Immortalization of human lymphocytes by co-cultivation with lethally irradiated autologous T-cell lines harbouring human T-cell leukaemia virus-I. J Cancer Res Clin Oncol. 1985;110(1):82–84. doi: 10.1007/BF00402508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- D'Onofrio C., Lohmann-Matthes M. L. Chemiluminescence of macrophages depends upon their differentiation stage: dissociation between phagocytosis and oxygen radical release. Immunobiology. 1984 Dec;167(5):414–430. doi: 10.1016/S0171-2985(84)80074-1. [DOI] [PubMed] [Google Scholar]
- D'Onofrio C., Paradisi F. In-vitro differentiation of human monocytes into mature macrophages during long-term cultures. Immunobiology. 1983 Feb;164(1):13–22. doi: 10.1016/S0171-2985(83)80013-8. [DOI] [PubMed] [Google Scholar]
- D'Onofrio C., Perno C. F., Mazzetti P., Graziani G., Calio' R., Bonmassar E. Depression of early phase of HTLV-I infection in vitro mediated by human beta-interferon. Br J Cancer. 1988 May;57(5):481–488. doi: 10.1038/bjc.1988.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vecchis L., Graziani G., Macchi B., Grandori C., Pastore S., Popovic M., Gallo R. C., Bonmassar E. Decline of natural cytotoxicity of human lymphocytes following infection with human T-cell leukemia/lymphoma virus (HTLV). Leuk Res. 1985;9(3):349–355. doi: 10.1016/0145-2126(85)90056-6. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Retroviruses as carcinogens and pathogens: expectations and reality. Cancer Res. 1987 Mar 1;47(5):1199–1220. [PubMed] [Google Scholar]
- Franchini G., Wong-Staal F., Gallo R. C. Human T-cell leukemia virus (HTLV-I) transcripts in fresh and cultured cells of patients with adult T-cell leukemia. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6207–6211. doi: 10.1073/pnas.81.19.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C. The human T-cell leukemia/lymphotropic retroviruses (HTLV) family: past, present, and future. Cancer Res. 1985 Sep;45(9 Suppl):4524s–4533s. [PubMed] [Google Scholar]
- Graziani G., Pasqualetti D., Lopez M., D'Onofrio C., Testi A. M., Mandelli F., Gallo R. C., Bonmassar E. Increased susceptibility of peripheral mononuclear cells of leukemic patients to HTLV-I infection in vitro. Blood. 1987 Apr;69(4):1175–1181. [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali R. A., Sarkar N. H., Whittington E., Good R. A. Prostaglandin regulation of murine mammary tumor virus production: a basis for some of the glucocorticoid and prolactin actions on mammary tumor cell cultures. Prostaglandins Leukot Med. 1982 Dec;9(6):641–655. doi: 10.1016/0262-1746(82)90021-x. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985 Apr;45(4):1437–1443. [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Manzari V., Gradilone A., Barillari G., Zani M., Collalti E., Pandolfi F., De Rossi G., Liso V., Babbo P., Robert-Guroff M. HTLV-I is endemic in southern Italy: detection of the first infectious cluster in a white population. Int J Cancer. 1985 Nov 15;36(5):557–559. doi: 10.1002/ijc.2910360507. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Matis L. A., Megson M., Bunn P. A., Murray C., Mann D. L., Gallo R. C., Broder S. Generation of an HLA-restricted cytotoxic T cell line reactive against cultured tumor cells from a patient infected with human T cell leukemia/lymphoma virus. J Exp Med. 1983 Sep 1;158(3):994–999. doi: 10.1084/jem.158.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Olsson I. L., Breitman T. R., Gallo R. C. Priming of human myeloid leukemic cell lines HL-60 and U-937 with retinoic acid for differentiation effects of cyclic adenosine 3':5'-monophosphate-inducing agents and a T-lymphocyte-derived differentiation factor. Cancer Res. 1982 Oct;42(10):3928–3933. [PubMed] [Google Scholar]
- Robert-Guroff M., Ruscetti F. W., Posner L. E., Poiesz B. J., Gallo R. C. Detection of the human T cell lymphoma virus p19 in cells of some patients with cutaneous T cell lymphoma and leukemia using a monoclonal antibody. J Exp Med. 1981 Dec 1;154(6):1957–1964. doi: 10.1084/jem.154.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. G., Benedetto A., Carruba G., Garaci E., Jaffe B. M. Prostaglandin A compounds as antiviral agents. Science. 1980 Aug 29;209(4460):1032–1034. doi: 10.1126/science.6157190. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Carruba G., Garaci E., Jaffe B. M., Benedetto A. Prostaglandins of the A series inhibit Sendai virus replication in cultured cells. J Gen Virol. 1981 Mar;53(Pt 1):75–83. doi: 10.1099/0022-1317-53-1-75. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Crisari A., Benedetto A., Amici C. Modulation of the growth of a human erythroleukemic cell line (K562) by prostaglandins: antiproliferative action of prostaglandin A. Cancer Res. 1986 Dec;46(12 Pt 1):6073–6077. [PubMed] [Google Scholar]
- Santoro M. G., Favalli C., Mastino A., Jaffe B. M., Esteban M., Garaci E. Antiviral activity of a synthetic analog of prostaglandin A in mice infected with influenza A virus. Arch Virol. 1988;99(1-2):89–100. doi: 10.1007/BF01311026. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Fukushima M., Benedetto A., Amici C. PGJ2, a new antiviral prostaglandin: inhibition of Sendai virus replication and alteration of virus protein synthesis. J Gen Virol. 1987 Apr;68(Pt 4):1153–1158. doi: 10.1099/0022-1317-68-4-1153. [DOI] [PubMed] [Google Scholar]
- Suciu-Foca N., Rubinstein P., Popovic M., Gallo R. C., King D. W. Reactivity of HTLV-transformed human T-cell lines to MHC class II antigens. Nature. 1984 Nov 15;312(5991):275–277. doi: 10.1038/312275a0. [DOI] [PubMed] [Google Scholar]
- Svec J., Svec P., Halcak L., Thurzo V. Role of natural prostaglandins in the control of murine mammary tumor virus expression. J Cancer Res Clin Oncol. 1982;103(1):55–67. doi: 10.1007/BF00410306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn R. M., Henney C. S. Kinetic analysis of target cell destruction by effector T cells. I. Delineation of parameters related to the frequency and lytic efficiency of killer cells. J Immunol. 1976 Dec;117(6):2213–2219. [PubMed] [Google Scholar]
- Volkman D. J., Popovic M., Gallo R. C., Fauci A. S. Human T cell leukemia/lymphoma virus-infected antigen-specific T cell clones: indiscriminant helper function and lymphokine production. J Immunol. 1985 Jun;134(6):4237–4243. [PubMed] [Google Scholar]
- Wong-Staal F., Gallo R. C. Human T-lymphotropic retroviruses. Nature. 1985 Oct 3;317(6036):395–403. doi: 10.1038/317395a0. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Guo H. G., Reitz M., Jr, Maluish A., Mitsuya H., Broder S. Alterations in cytotoxic and helper T cell function after infection of T cell clones with human T cell leukemia virus, type I. J Clin Invest. 1986 May;77(5):1466–1473. doi: 10.1172/JCI112459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M. Expression of the HTLV-1 genome and its association with a unique T-cell malignancy. Biochim Biophys Acta. 1987 Jul 8;907(2):145–161. doi: 10.1016/0304-419x(87)90003-5. [DOI] [PubMed] [Google Scholar]



